Abstract
Malignant transformation of endometriosis is an infrequent complication. Clear cell carcinoma from endometriosis is very rare in the paraovarian cyst. To date no cases have been reported. We report a case of clear cell carcinoma arising from endometriosis of the paraovarian cyst with a brief review of literature.
Since the first description of adenocarcinoma arising in the setting of endometriosis in 1925 by Sampson,1 the malignant transformation of endometriosis has been described in numerous case reports and review of the literatures. About 0.7-1.0% of patients with endometriosis have lesions that undergo malignant transformation.2,3
In 79% of the cases, the ovary is the primary site of malignancy, whereas extragonadal sites represent 21% of tumors.4 There has been 1 documented case of clear cell carcinoma arising in vulva endometriosis.5 Clear cell carcinoma from endometriosis is very rare in the paraovarian cyst and to date no cases have been reported. We report a case of clear cell carcinoma from endometriosis of the paraovarian cyst with a brief review of literatures.
A 62-year old, para 3, married female consulted to our institution in May 2008 with complaints of abdominal discomfort for 1 month. A pelvic examination revealed a normal sized uterus and large pelvic mass. Computed tomography showed a 16 cm sized cystic mass on the right adnexa with enhanced solid component (Fig. 1). Her past medical and surgical history was unremarkable. She experienced menopause at 49 years old and did not take any hormone replacement therapy.
Preoperative laboratory studies revealed the following values: Hb 10 g/dl, WBC count 4,600/mm3, platelet count 324,000/mm3. The results of other investigations, including urinalysis, liver function tests, renal function tests, electrolytes, chest radiography, and electrocardiography revealed no abnormalities. Tumor markers were slightly elevated as follows; CA125, 67 U/ml (0-48); CA19-9, 58 U/ml (0-37); CEA, <1.0 ng/ml (0-5).
At the time of surgery, the pelvic mass was a cystic lesion adhesive to the right salpinx, uterine fundus, rectum and right retroperitoneum. Cyst surface was clear and well marginated with serous fluid inside. Uterus was bicornuate shaped and both adnexae were grossly free. There was no suspicious malignant lesions in the pelvis by inspection and palpation. Under the impression of a paraovarian cyst, cyst excision and washing cytology was done. There was minimal intraoperative spillage. The patient had an uncomplicated post-operative course.
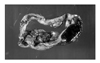
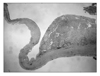
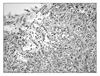
On pathologic gross examination, there was a 12×7.5×5.5 cm cyst with white colored capsule and no infiltration of the capsule. A unilocular cyst was seen with focal areas of solid papillary growth. The remaining internal lining surface showed areas of hemorrhage (Fig. 2). Microscopic findings revealed a clear cell carcinoma arising from endometriosis with localization in the inner side of the capsule. There was no endolymphatic tumor emboli. The cyst wall contained a smooth muscle layer and the internal surface was focally lined by cuboidal to columnar epithelium and endometrial stromal cells. This suggested an endometriosis arising from the uterine adnexa (Fig. 3). Higher power magnification of the solid portion revealed tumor cells with pleomorphic nuclei and eosinophilic or clear cytoplasm arranged in tubular or acinar structures. A hobnail pattern was prominent. This coincided with the typical clear cell morphology (Fig. 4).
The patient underwent re-operation for surgical staging with total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, pelvic lymph node dissection, appendectomy, and multiple random peritoneal biopsies. On pathologic examination, there was no tumor in any specimens. Endometriosis was found in the right ovary. Post-operative hospital course was uneventful and the decision was made to recommend adjuvant chemotherapy with paclitaxel and carboplatin.
Malignant transformation of endometriosis in gonadal and extragonadal sites have been well documented since Sampson reported the first case in 1925.1 The criteria presented by Sampson for diagnosis of a malignancy arising in endometriosis are: 1) demonstration of both cancerous and benign endometrial tissue in the same ovary, 2) demonstration of cancer arising in the tissue and not invading it from another source, and 3) presence of tissue resembling endometrial stroma surrounding characteristic epithelial glands.1
The estimated prevalence of endometriosis are 15% of premenopausal and 2-5% of postmenopausal women.6 However, about 0.7-1.0% of patients with endometriosis have lesions that undergo malignant transformation.2,3 Heap et al.4 reviewed 205 reported cases of malignancy arising in endometriosis and reported that the ovary was the most frequently involved, accounting for 165 cases (78.7%), and that extragonadal tumors were present in 44 (21.3%). The rectovaginal septum, colon, vagina, and pelvic peritoneum represented the majority of extragonadal sites. Irvin et al.7 also reviewed 222 reported cases of malignancy arising in endometriosis, and reported that the ovary was most frequently involved, accounting for 169 cases (76%), and extragonadal tumors were 53 (24%). This concords with the frequency of endometriosis according to location.
Pelvic pain or pelvic mass in a postmenopausal woman with a previous history of endometriosis should raise suspicions of reactivation or malignant transformation of endometriosis. Vaginal bleeding may signify the presence of a vaginal or rectovaginal septum lesion, and the bleeding may be caused by estrogenic stimulation. Malignant transformation of colorectal endometriosis may produce gastrointestinal dysfunction and/or bleeding.8-10 Urinary symptoms may herald urinary tract involvement with this disease.11,12
When malignant transformation occurs in endometriosis, it tends to be discovered at an early stage and is often of low grade. Primary surgical treatment with complete resection of pelvic tumors should be performed when feasible. Appropriate staging biopsies of lymph nodes and tissues in the upper abdomen should be performed when macroscopic disease is confined to pelvis.4
Aure et al.13 noted that the prognosis and 5-year survival were the same, stage for stage, as those for patients with ovarian carcinoma. Malignant transformation within endometriomas or within extragonadal endometriosis confined to the genital tract carries a much better prognosis, with a 67% 5-year survival for those with disease confined to the ovary and 100% 5-year survival for those with extragonadal disease confined to the site of the origin. Disseminated intraperitoneal disease had a poor prognosis, with a 12% 5-year survival.4
Among malignancies arising from endometriosis of the ovaries or extragonadal lesions, endometrioid adenocarcinoma was the most common histologic type (69.1%). Clear cell histology was seen in only 4.5% of extragonadal malignancies.4 To our knowledge, this is the first case of clear cell carcinoma arising from endometriosis of the paraovarian cyst.
Figures and Tables
Fig. 1
Computed tomography shows 16 cm sized huge cystic mass with small solid portion (arrow) in the pelvic cavity.
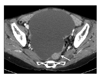
Fig. 2
A unilocular cyst is seen with focal areas of solid papillary growth. The remaining internal lining surface shows areas of hemorrhage.
References
1. Sampson JA. Endometrial carcinoma of ovary, arising endometrial tissue in that organ. Arch Surg. 1925. 10:1–72.
2. Corner GW, Hu CY, Hertig AT. Ovarian carcinoma arising in endometriosis. Am J Obstet Gynecol. 1950. 59:760–774.
3. Lauslahti K. Malignant external endometriosis: a case of adenocarcinoma of umbilical endometriosis. Acta Pathol Microbiol Scand Suppl. 1972. 233:98–102.
4. Heaps JM, Nieberg RK, Berek JS. Malignant neoplasm arising in endometriosis. Obstet Gynecol. 1990. 75:1023–1028.
5. Cho HJ, Lee KJ, Cha SH, Seong SJ, Park CT, Lee KH, et al. Clear cell carcinoma of the vulva arising in endometriosis. Korean J Obstet Gynecol. 2003. 46:847–850.
6. Punnonen R, Klemi PJ, Nikkanen V. Postmenopausal endometriosis. Eur J Obstet Gynecol Reprod Biol. 1980. 11:195–206.
7. Irvin W, Pelkey T, Rice L, Andersen W. Endometrial stromal sarcoma of the vulva arising in extraovarian endometriosis: a case report and literature review. Gynecol Oncol. 1998. 71:313–316.
8. Reintoft I, Lange AP, Skipper A. Coincidence of granulosa-cell tumour of ovary and development of carcinoma in rectal endometriosis. Acta Obstet Gynecol Scand. 1974. 53:185–189.
9. Amano S, Yamada N. Endometrioid carcinoma arising from endometriosis of the sigmoid colon: a case report. Hum Pathol. 1981. 12:845–848.
10. Amano S, Yamada N. Endometrioid carcinoma arising from endometriosis of the sigmoid colon: a case report. Hum Pathol. 1981. 12:845–848.
11. Shamsuddin AK, Villa Santa U, Tang CK, Mohamed NC. Adenocarcinoma arising from extragonadal endometriosis 14 years after total hysterectomy and bilateral salpingo-oophorectomy for endometriosis: report of a case with ultrastructural studies. Am J Obstet Gynecol. 1979. 133:585–586.
12. Kapadia SB, Russak RR, O'Donnell WF, Harris RN, Lecky JW. Postmenopausal ureteral endometriosis with atypical adenomatous hyperplasia following hysterectomy, bilateral oophorectomy, and long-term estrogen therapy. Obstet Gynecol. 1984. 64:3 Suppl. 60S–63S.
13. Aure JC, Hoeg K, Kolastad P. Carcinoma of the ovary and endometriosis. Acta Obstet Gynecol Scand. 1971. 50:63–67.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download