Abstract
As a key regulator of melanogenesis, p53 controls microphthalmia-associated transcription factor (MITF) and tyrosinase expression. The anti-oxidant enzyme heme oxygenase-1 (HO-1) is induced by various forms of cellular stress and diverse oxidative stimuli. However, few studies have examined the role of HO-1 in melanogenesis. Therefore, the aim of this study was to determine the role of HO-1 in melanogenesis and the mechanism underlying this relationship. Cultures of normal human melanocytes were treated with the HO-1 inducer cobalt protoporphyrin (CoPP) or the HO-1 inhibitor zinc protoporphyrin (ZnPP). We then measured the melanin content of the cells. Additional analyses consisted of Western blotting and RT-PCR. The results showed that the cellular melanin content was increased by CoPP and decreased by ZnPP. The Western blot and RT-PCR analyses showed that CoPP increased p53, MITF and tyrosinase levels, and ZnPP reduced all of them. The knockdown of p53 by siRNA transfection was followed by large decreases in the expression levels of p53, MITF and tyrosinase at 3 h of transfection. The presence of CoPP or ZnPP had no significant increased or decreased effects on MITF and tyrosinase levels from 15 h in the siRNA transfectants. Our results suggest that HO-1 modulates melanogenesis in human melanocytes via a p53-dependent pathway.
Melanogenesis in the skin is regulated by various factors, among which, tyrosinase is a key enzyme. The expression of tyrosinase is induced by the microphthalmia-associated transcription factor (MITF).1 p53 also plays a crucial role in melanogenesis, not only through ultraviolet (UV)-induced pigmentation via reactive oxygen species (ROS), such as H2O2, which induces p53 via the NF-kB pathway, but also through non-UV-induced pigmentation, such as inflammatory or post-inflammatory hyperpigmentation.2 Heme oxygenase (HO)-1 (also known as heat shock protein 32), the inducible 32-kDa isoform of the enzyme HO, is strongly induced in response to cellular stress and diverse oxidative stimuli, including its heme substrate, heat shock, UV irradiation, ROS, nitric oxide, inflammatory cytokines, prostaglandins, ethanol, heavy metals, and hypoxia.3 HO-1 protects human melanocytes from oxidative stress, in the form of UV or other endogenous and exogenous stimuli, via the nuclear factor E2-related factor 2 (Nrf2)-antioxidant response element (ARE) pathway.4 It has thus generated interest as a therapeutic target in various oxidative-stress-associated diseases and tumors.356 The transcription factor p53 not only is a powerful tumor suppressor but also plays a central role in skin pigmentation.7 UV is a potent inducer of hyperpigmentation in normal melanocytes, and p53 is a target of UV.8 A few studies have suggested an association between p53 expression and HO-1 activity.91011 However, little is known about the role of HO-1 in melanogenesis. Therefore, in this study, we investigated the effect of HO-1 on melanin production in normal human melanocytes and the role of p53 in HO-1-related melanogenesis.
Primary cultures of normal human melanocytes were established from neonatal foreskin as described previously.12 The cells were suspended in Medium 254 (#M-254-500, Life Technologies, Grand Island, NY, USA) supplemented with human melanocyte growth supplement (HMGS; #S-002-5, Life Technologies) and incubated at 37℃ in a 5% CO2 incubator with 99% humidity. Melanocytes from the third or fourth passage were used in the experiments. The HO-1 activator protoporphyrin IX cobalt chloride (CoPP) and the HO-1 inhibitor protoporphyrin IX zinc (ZnPP)1 were purchased from Sigma Aldrich Chemical Co. (#C1900 for CoPP; #282820 for ZnPP; St. Louis, MO, USA).
The cell viability of CoPP and ZnPP towards primary cultures of normal human melanocytes was determined by a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. The melanin contents of CoPP- and ZnPP-treated cultures were then measured by ELISA, based on an absorbance of 490 nm and compared against a standard curve generated using synthetic melanin (Sigma Aldrich).
Protein levels were examined by Western blot analysis of HO-1 (#API-SPA-895, 1:2000, Stressgen, Victoria, BC, Canada), tyrosinase (#05-647, 1:1000, Upstate, Lake Placid, NY, USA), MITF (#12590, 1:1000, Cell Signaling, Danvers, MA, USA), and p53 (#sc-126, 1:1000, Santa Cruz, Dallas, TX, USA) after CoPP and ZnPP treatments. Briefly, cultured normal human melanocytes were lysed in RIPA buffer [1% Triton X-100, 150 mM NaCl, 0.1% SDS, 50 mM Tris-HCl (pH 7.5), 2 mM EDTA]. Cell extracts (30 µg each) in SDS sample buffer (50 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 0.1% bromophenol blue, and 5% β mercaptoethanol) were separated by SDS-PAGE on a 12% polyacrylamide gel and transferred to a PVDF membrane (0.45 µm). After incubation of the membrane with blocking solution [5% skim milk in TBS with 0.1% Tween-20 (TBS-T)] for 1 h at room temperature, the protein bands were probed with anti-HO-1, anti-p53, anti-tyrosinase, and anti-MITF antibodies overnight at 4℃. Peroxidaseconjugated AffiniPure F(ab')2 fragment goat anti-rabbit IgG (1:4000 in 0.5% skim milk in TBS-T; Jackson Immunoresearch, West Grove, PA, USA) and peroxidase-conjugated AffiniPure F(ab')2 fragment goat anti-mouse IgG (Jackson Immunoresearch) were used as the secondary antibodies. The immune complexes were visualized using an enhanced chemiluminescence kit (Millipore, Billerica, MA, USA). The obtained chemiluminescent signals were scanned by the ImageQuant LAS 4000 Mini (Fujifilm, Tokyo, Japan) and analyzed with an image analysis program (Multi Gauge Ver. 3.0, Fujifilm).
The expression of p53, HO-1, tyrosinase, and MITF mRNA in ZnPP-treated cultures was examined by RT-PCR to determine whether the inhibitor-induced decrease in melanin production was dependent on the p53 pathway. Cells were seeded in 60-mm culture dishes at 1×105/dish and grown to confluency. Total cellular RNA was extracted using the RNeasy mini kit (Qiagen Inc., GmbH, Hilden, Germany); cDNA was obtained using the Improm-II reverse transcription system (Promega, Madison, WI, USA) and analyzed by PCR using the HotStarTaq master mix kit (Qiagen Inc.). The following oligonucleotide PCR primers were synthesized by Bioneer Co. (Daejeon, South Korea):
HO-1, sense 5'-CAGGCAGAGAATGCTGAGTTC-3' and antisense 5'-GATGTTGAGCAGGAACGCAGT-3' p53, sense 5'- ATCGTGGAGGCATGAGCAGA-3' and antisense 5'- TCTGGAGTTTCTGCTGCTGCTA -3' MITF, sense 5'-CCGTCTCTCACTGGATTCGTG -3' and antisense 5'-CGTGAATGTGTGTTCATGCCTGG-3' Tyrosinase, sense 5'-CTCCGCTGGCCATTTCCCTA -3' and antisense 5'-GGTGCTTCATGGGCAAAATC-3' GADPH (control), sense 5'- ACTTCAACAGCGACACCCACTC-3' and antisense 5'- CTTCCTCTTGTGCTCTTGCTGG-3'
PCR amplification was performed using the Palm-Cycler (Corbett Research, Sydney, Australia). The parameters for HO-1 were as follows: 25 cycles at 94℃ for 30 s (denaturation), 59℃ for 30 s (annealing), and 72℃ for 1 min (elongation). Those for tyrosinase were 25 cycles at 94℃ for 30 s (denaturation), 58℃ for 30 s (annealing), and 72℃ for 1 min (elongation). For MITF, the following parameters were used: 25 cycles at 94℃ for 30 s (denaturation), 55℃ for 30 s (annealing), and 72℃ for 1 min (elongation). For the GADPH control, the parameters were 25 cycles at 94℃ for 30 s, 63℃ for 30 s, and 72℃ for 1 min. The PCR products were electrophoresed on 1.2% agarose gels. Each DNA band was visualized by staining with SYBR Safe DNA gel stain (#S33102, Invitrogen, Carlsbad, CA, USA). The obtained chemiluminescent signals were scanned by the ImageQuant LAS 4000 Mini (Fujifilm, Tokyo, Japan) and analyzed with an image analysis program (Multi Gauge Ver. 3.0, Fujifilm).
The cells were transfected with the SignalSilence p53 siRNA (#12590, Cell Signaling) using Lipofectamine RNAiMAX transfection reagent (#133778, Invitrogen, Carlsbad, CA, USA). The siRNA transfectants were incubated at 37℃ in a CO2 incubator for 48 h, after which the efficacy of gene knockdown was determined. HO-1, p53, MITF, and tyrosniase protein levels were analyzed by Western blot analysis and p53, MITF, tyrosinase mRNA expressions by RT-PCR. The obtained chemiluminescent signals were scanned by the ImageQuant LAS 4000 Mini (Fujifilm, Tokyo, Japan) and analyzed with an image analysis program (Multi Gauge Ver. 3.0, Fujifilm).
All experiments were performed at least three times. The results are expressed as the mean±standard deviation (SD). Statistical analysis was performed using SPSS (ver. 21.0; IBM Corp., NY, USA). We used Pearson's correlation analysis in an MTT assay, melanin content assay, and Western blot analysis of ZnPP dose-dependent treatments. The ANCOVA test was used in the analysis of time-course expressions between control and CoPP or ZnPP treatment groups. A p value<0.05 was considered to indicate statistical significance.
The MTT assay showed marked decrease of cell viability in a dose-dependent manner in the ZnPP treatment groups compared with control which was not treated with ZnPP (p=0.016), and cell viability at 20 µM decreased below 50%. However, the results were not statistically significant in the CoPP treatment groups compared with the control which was not treated with CoPP (p=0.081; Fig. 1). Therefore, the treatment dose, determined by the MTT assay, was 10 µM for both CoPP and ZnPP. The effect of HO-1 on melanin production in normal human melanocytes was screened by ELISA to determine the melanin content of the cells. The results showed that the melanin content was increased by treating with CoPP compared with the control which was not treated with CoPP (p=0.018; Fig. 2A), however it decreased markedly by treating with ZnPP in a dose-dependent manner compared with control which was not treated with ZnPP (p=0.012; Fig. 2B).
Western blot analysis revealed marked dose-dependent reductions in p53, tyrosinase, and MITF protein expressions in cells treated with ZnPP (Fig. 3A). Scanned chemiluminescent signals were analyzed with the image analysis program, and the data was normalized with β-actin. The results showed only the increase in HO-1 expression was statistically significant according to the treatment doses of ZnPP compared to the control group (p=0.043; Fig. 3B). CoPP (10 µM) treatments increased expressions of p53 (p=1.000), MITF (p=0.095), and tyrosinase (p=0.748), but not statistically significantly (Fig. 3C, D). The decrease in p53 (p=0.045), tyrosinase (p=0.025), and MITF (p=0.460) in the ZnPP 10 µM-treated cultures continued until 72 h after treatment (Fig. 3C, D). The increase in HO-1 protein expression increased after 15 h in cultures treated with CoPP (p=0.105) and ZnPP (p=0.087).
The Western blot analysis of normal human melanocytes transfected with p53 siRNA (Fig. 4). HO-1, p53, MITF, and tyrosinase levels decreased markedly and progressively from 3 h post-transfection. There were no increases in MITF and tyrosinase proteins in response to CoPP treatment after transfection (Fig. 4A). Scanned chemiluminescent signals were analyzed with the image analysis program, and data was normalized with β-actin (Fig. 4B). p53 (p=1.000), MITF (p=0.802), and tyrosinase (p=0.150) expressions in CoPP treatments after transfection were not significant, but only change in HO-1 expression was statistically significant compared with control groups which were not pre-transfected before the CoPP treatment (p=0.019). There was a slight decrease in MITF, but no decrease in tyrosinase proteins in response to the HO-1 inhibitor ZnPP after transfection (Fig. 4C). The intensity bar graphs showed only MITF (p=0.032) expressions in the ZnPP treatment groups were statistically significant, compared with the control groups which were not pre-transfected before the ZnPP treatment (Fig. 4D). However, HO-1 (p=0.137) and tyrosinase (p=0.138) expressions were not significant.
RT-PCR showed similar results for the corresponding mRNA levels after p53 siRNA transfection (Fig. 5). p53, MITF and tyrosinase mRNA levels decreased at 3 h and 15 h post-transfection and CoPP treatment (Fig. 5A). Scanned chemiluminescent signals were analyzed with the image analysis program, and the data was normalized with GAPDH (Fig. 5B). Only the decrease in p53 expression from the CoPP treatment groups after transfection was statistically significant compared with the control groups which were not pre-transfected before the CoPP treatment (p=0.014). There were no statistically significant increases in MITF (p=0.648) and tyrosinase (p=0.793) mRNAs in response to CoPP from 24 h. In ZnPP treatments, p53 and tyrosinase mRNA levels decreased at 3 h and 15 h post-transfection and ZnPP treatment (Fig. 5C). Scanned chemiluminescent signals were analyzed with the image analysis program. Each intensity bar was normalized with GAPDH (Fig. 5D). There were no decreases in MITF and tyrosinase mRNAs from 15 h and 24 h in response to ZnPP after transfection. The changes were not statistically significant for p53 (p=0.318), MITF (p=0.713), and tyrosinase (p=0.827) expressions.
HO-1 is a phase II antioxidant produced in the skin in response to oxidative stress. However, little is known about its role in melanin production. Elassiuty et al.13 showed that HO-1 is up-regulated in UV-exposed vitiligo skin and non-lesional vitiligo melanocyte cultures. They suggested that HO-1 protects melanocytes and is related to lesional repigmentation following UV exposure. We measured the protein levels of tyrosinase in healthy melanocytes treated with CoPP and ZnPP. Our results showed that melanogenesis is inhibited by the HO-1 inhibitor ZnPP via the suppression of tyrosinase and MITF.
Marrot et al.14 reported that solar UV exposure increases HO-1 and p53 levels in human melanocytes from Caucasians, but whether HO-1 activity is related to p53 expression was not determined. In our study, p53 expression was responsive to HO-1 activity, in that CoPP induced and ZnPP suppressed p53 expression. Lee et al.9 reported the concurrent expression of HO-1 and p53. In the Western blot analysis of human retinal pigment epithelial cells, the authors also observed that CoPP and ZnPP increased HO-1 and p53 expression in dose- and time-dependent manners. On the other hand, ZnPP decreased HO-1 activity, while increasing HO-1 protein expression. In our experiments, both CoPP and ZnPP increased HO-1 expression in the Western blot analysis, showing results similar to their study.9 The increased HO-1 protein levels from ZnPP in retinal pigment epithelial cells and our normal human melanocytes might be explained by the HO-1 protein levels increasing over time after decreased HO-1 activity as a rebound or compensation phenomenon. They therefore suggested that HO-1 activity is associated with the regulatory mechanisms of p53 expression in retinal cells. Our study demonstrated that HO-1 controls p53 expression in normal human melanocytes and that the modulation of melanogenesis by HO-1 is dependent on p53. Nam and Sabapathy10 also found that p53 is a target of HO-1. They suggested that p53 is involved in the increase in HO-1 triggered by oxidative stress that develops in response to H2O2. Taken together, these results suggest that HO-1 interacts with the p53 pathway.
Both UV and H2O2 exposure can induce p53.78 In its initiation of melanogenesis, p53 regulates the expression of the transcription factor hepatocyte nuclear factor-1α, which in turn regulates MITF and tyrosinase levels.2 In this study, HO-1 induced p53, which then increased MITF and tyrosinase. The knockdown of p53 by siRNA transfection resulted in a decrease in MITF and tyrosinase within 3 h, followed by the slow recovery of its expression, at both the mRNA and protein levels, beginning at 15 h posttransfection. The suppression of tyrosinase lasted longer than that of MITF. These results suggest that HO-1-induced melanogenesis is mediated by p53, which stimulates both MITF and tyrosinase expression, which is shown as a summarized schematic diagram (Fig. 6). The finding that tyrosinase and MITF expression was refractory to an inducer and inhibitor of HO-1 after p53 knockdown provided further evidence of the dependence of HO-1 on p53 in melanogenesis. Based on these observations, HO-1 inhibitors that decrease melanin production could serve as effective therapeutic agents for the treatment of skin diseases characterized by hyperpigmentation, such as melasma and post-inflammatory hyperpigmentation. Further experiments are warranted to elucidate the exact nature of the interaction between p53 and HO-1 in melanogenesis.
Figures and Tables
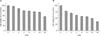 | FIG. 1MTT cell viability assay. (A) The HO-1 inducer CoPP and (B) the HO-1 inhibitor ZnPP treatments show decrease of cell viability in a dose-dependent manner. The results are not statistically significant in CoPP treatment groups compared with control (p=0.081), but marked decrease of cell viability at 20 µM below 50% in ZnPP treatment groups compared with control (p=0.016). C: control. |
 | FIG. 2Measurement of melanin content in normal human melanocytes. (A) CoPP increased (p=0.018) and (B) ZnPP decreased (p=0.012) cellular melanin content in a dose-dependent manner compared with control. C: control. |
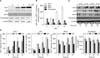 | FIG. 3Western blot analysis. (A) ZnPP decreases p53, MITF, and tyrosinase expressions in normal human melanocytes in a dose-dependent manner. (B) Scanned chemiluminescent signals were analyzed with the image analysis program. Each bar represents band intensity normalized with that of β-actin. Only increase in HO-1 expression is statistically significant according to the treatment doses of ZnPP compared to control group (p=0.043). (C) CoPP (10 µM) treatments increase expressions of p53, MITF, and tyrosinase, and ZnPP (10 µM) treatments decrease expressions in p53, tyrosinase, and MITF until 72 h after treatment. (D) Scanned chemiluminescent signals were analyzed with an image analysis program. Each intensity bar represents normalized with β-actin. Only decreases in p53 (p=0.045) and tyrosinase (p=0.025) expressions upon ZnPP treatment are statistically significant compared with control groups. All experiments were performed at least three times independently, and representative results are shown. The data represent mean±SD. C: control, CP: CoPP, ZP: ZnPP, *Statistical significance (p<0.05). |
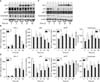 | FIG. 4Western blot analysis in normal human melanocytes transfected with p53 siRNA. (A) HO-1, p53, MITF, and tyrosinase levels decrease from 3 h post-transfection. There are no increases in MITF and tyrosinase proteins in response to the HO-1 inducer CoPP treatment after transfection. (B) Scanned chemiluminescent signals were analyzed with the image analysis program. Each bar represents band intensity normalized with that of β-actin. Only HO-1 expressions by CoPP treatment groups after transfection are statistically significant compared with control groups without transfection (p=0.019). C: CoPP treatment, T: transfection and CoPP treatment. (C) HO-1, p53, MITF, and tyrosinase levels decreased markedly and progressively 3 h post-transfection. (D) Scanned chemiluminescent signals were analyzed with an image analysis program. Each bar represents band intensity normalized with that of β-actin. There are slight decrease in MITF, but no decrease in tyrosinase in response to the HO-1 inhibitor ZnPP after transfection. Only MITF (p=0.032) expressions by ZnPP treatment groups after transfection are statistically significant compared with control groups without transfection. C: ZnPP treatment, T: transfection and ZnPP treatment. All experiments were performed at least three times independently, and representative results are shown. The data represent mean±SD. *Statistical significance (p< 0.05). |
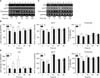 | FIG. 5RT-PCR analysis after p53 siRNA transfection. (A) MITF and tyrosinase mRNA levels decreased 3 h and 15 h post-transfection and CoPP treatment. (B) Scanned chemiluminescent signals were analyzed with the image analysis program. Each bar represents band intensity normalized with that of GAPDH. Only p53 expressions by CoPP treatment groups after transfection are statistically significant compared with control groups without transfection (p=0.014). There are no increases in MITF and tyrosinase mRNAs in response to the HO-1 inducer CoPP from 24 h. C: CoPP treatment, T: transfection and CoPP treatment. (C) p53 and tyrosinase mRNA levels decrease at 3 h and 15 h post-transfection and ZnPP treatment. (D) Scanned chemiluminescent signals were analyzed with the image analysis program. Each bar represents band intensity normalized with that of GAPDH. There are no decreases MITF and tyrosinase mRNAs from 15 h and 24 h, respectively, in response to the HO-1 inhibitor ZnPP treatments after transfection. All expressions are not statistically significant. C: ZnPP treatment, T: transfection and ZnPP treatment. All experiments were performed at least three times independently, and representative results are shown. The data represent mean±SD. *Statistical significance (p<0.05). |
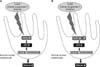 | FIG. 6Modulation of melanogenesis by HO-1 via p53 in normal human melanocytes. (A) HO-1 inducer, CoPP, increases melanin production by inducing p53, which in turn stimulates both MITF and tyrosinase expression. (B) HO-1 inhibitor, ZnPP, decreases melanin production by suppressing p53 which decreases both MITF and tyrosinase expression. |
References
1. Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999; 104:1631–1639.

2. Schallreuter KU, Kothari S, Chavan B, Spencer JD. Regulation of melanogenesis--controversies and new concepts. Exp Dermatol. 2008; 17:395–404.
3. Zhao H, Ozen M, Wong RJ, Stevenson DK. Heme oxygenase-1 in pregnancy and cancer: similarities in cellular invasion, cytoprotection, angiogenesis, and immunomodulation. Front Pharmacol. 2015; 5:295.

4. Jian Z, Li K, Liu L, Zhang Y, Zhou Z, Li C, et al. Heme oxygenase-1 protects human melanocytes from H2O2-induced oxidative stress via the Nrf2-ARE pathway. J Invest Dermatol. 2011; 131:1420–1427.

5. Jin SA, Park JJ, Lee JB, Lee SC, Yun SJ. Decreased heme oxygenase-1 expression distinguishes human melanomas from melanocytic nevi. Pigment Cell Melanoma Res. 2010; 23:841–844.

6. Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000; 60:1121–1128.

7. Box NF, Terzian T. The role of p53 in pigmentation, tanning and melanoma. Pigment Cell Melanoma Res. 2008; 21:525–533.

8. Park HY, Kosmadaki M, Yaar M, Gilchrest BA. Cellular mechanisms regulating human melanogenesis. Cell Mol Life Sci. 2009; 66:1493–1506.

9. Lee SY, Jo HJ, Kim KM, Song JD, Chung HT, Park YC. Concurrent expression of heme oxygenase-1 and p53 in human retinal pigment epithelial cell line. Biochem Biophys Res Commun. 2008; 365:870–874.

10. Nam SY, Sabapathy K. p53 promotes cellular survival in a context-dependent manner by directly inducing the expression of haeme-oxygenase-1. Oncogene. 2011; 30:4476–4486.

11. Kim DH, Song NY, Kim EH, Na HK, Joe Y, Chung HT, et al. 15-deoxy-Δ12,14-prostaglandin J2 induces p53 expression through Nrf2-mediated upregulation of heme oxygenase-1 in human breast cancer cells. Free Radic Res. 2014; 48:1018–1027.

12. Eisinger M, Lee JS, Hefton JM, Darzynkiewicz Z, Chiao JW, de Harven E. Human epidermal cell cultures: growth and differentiation in the absence of differentiation in the absence of dermal components or medium supplements. Proc Natl Acad Sci U S A. 1979; 76:5340–5344.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download