Abstract
Objective
IL-1β is involved in the degradation of articular cartilage in various arthritides, including osteoarthritis (OA). Competitive inhibition of IL-1β by IL-1 receptor antagonists (IL-1Ra) may represent a pathogenesis-based strategy for inhibiting degradation of the cartilage matrix. We investigated the hypothesis that controlled release of IL-1Ra using injectable, thermoreversible and complex coacervate combination gels as drug delivery systems might reduce matrix degradation in OA.
Methods
Thermoreversible combination gels that can be injected into joints were formed in aqueous solution by making a complex coacervate with recombinant human IL-1Ra (anakinra) and cationic macromolecules, and this was followed by co-formulation with methylcellulose as a negative thermosensitive polysaccharide. Gels containing anakinra were positioned in the upper insert of a transwell system and human OA chondrocytes were placed in the lower compartment and then they were stimulated with IL-1β. The expression of matrix metalloproteinases (MMPs) was examined by performing real time PCR and ELISA.
Results
Complex coacervation between anakinra and protamine was successfully completed. IL-1Ra was released from the gels in a sustained release pattern for extended periods with minimal initial bursts. IL-1β markedly enhanced the expression of MMP. The IL-1Ra released from the gels significantly inhibited the IL-1β-induced MMP expression in the chondrocytes.
Conclusion
We developed and optimized a novel injectable and thermoreversible gel system for the controlled release of IL-1Ra, and this drug delivery system effectively inhibited the IL-1β-induced MMP expression of chondrocytes in a transwell system. Intraarticular local delivery of injectable and thermoreversible gels containing IL-1Ra into knees has the potential to provide prolonged therapy based on the pathophysiology of knee OA.
References
2. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000; 43:1905–15.
3. Caron JP, Fernandes JC, Martel-Pelletier J, Tardif G, Mineau F, Geng C, et al. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collage-nase-1 expression. Arthritis Rheum. 1996; 39:1535–44.

4. Pelletier JP, Caron JP, Evans C, Robbins PD, Georgescu HI, Jovanovic D, et al. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997; 40:1012–9.

5. Fernandes J, Tardif G, Martel-Pelletier J, Lascau-Coman V, Dupuis M, Moldovan F, et al. In vivo transfer of in-terleukin-1 receptor antagonist gene in osteoarthritic rab-bit knee joints: prevention of osteoarthritis progression. Am J Pathol. 1999; 154:1159–69.
6. Zhang X, Mao Z, Yu C. Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 receptor antagonist and interleukin-10. J Orthop Res. 2004; 22:742–50.

7. Haupt JL, Frisbie DD, McIlwraith CW, Robbins PD, Ghivizzani S, Evans CH, et al. Dual transduction of in-sulin-like growth factor-I and interleukin-1 receptor antagonist protein controls cartilage degradation in an osteoarthritic culture model. J Orthop Res. 2005; 23:118–26.
8. Wang HJ, Yu CL, Kishi H, Motoki K, Mao ZB, Muraguchi A. Suppression of experimental osteoarthritis by adenovirus-mediated double gene transfer. Chin Med J (Engl). 2006; 119:1365–73.

9. Chevalier X, Giraudeau B, Conrozier T, Marliere J, Kiefer P, Goupille P. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol. 2005; 32:1317–23.
10. Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004; (427 Suppl):S27–36.

11. Goldring MB, Fukuo K, Birkhead JR, Dudek E, Sandell LJ. Transcriptional suppression by interleukin-1 and in-terferon-gamma of type II collagen gene expression in human chondrocytes. J Cell Biochem. 1994; 54:85–99.
12. Taskiran D, Stefanovic-Racic M, Georgescu H, Evans C. Nitric oxide mediates suppression of cartilage proteogly-can synthesis by interleukin-1. Biochem Biophys Res Commun. 1994; 200:142–8.
13. Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002; 13:323–40.

14. Slack J, McMahan CJ, Waugh S, Schooley K, Spriggs MK, Sims JE, et al. Independent binding of interleukin-1 alpha and interleukin-1 beta to type I and type II interleukin-1 receptors. J Biol Chem. 1993; 268:2513–24.

15. Iqbal I, Fleischmann R. Treatment of osteoarthritis with anakinra. Curr Rheumatol Rep. 2007; 9:31–5.

16. Xia J, Dubin PL. Protein-polyelectrolyte complexes. Dubin PL, Bock J, Davis R, Schulz DN, Thies C, editors. Macromolecular Complexes in Chemistry and Biology. p. 247–71. Berlin: Springer-Verlag Telos;1994.

17. Poznansky MJ, Juliano RL. Biological approaches to the controlled delivery of drugs: a critical review. Pharmacol Rev. 1984; 36:277–336.
18. Gombotz WR, Pettit DK. Biodegradable polymers for protein and peptide drug delivery. Bioconjug Chem. 1995; 6:332–51.

19. Jin KM, Kim YH. Injectable, thermoreversible and complex coacervate combination gels for protein drug delivery. J Control Release. 2008; 127:249–56.

20. Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002; 46:2648–57.

21. Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005; 52:128–35.
22. Tetlow LC, Adlam DJ, Woolley DE. Matrix metal-loproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001; 44:585–94.

23. Qvist P, Bay-Jensen AC, Christiansen C, Dam EB, Pastoureau P, Karsdal MA. The disease modifying osteoarthritis drug (DMOAD): Is it in the horizon? Pharmacol Res. 2008; 58:1–7.

24. Abramson SB, Yazici Y. Biologics in development for rheumatoid arthritis: relevance to osteoarthritis. Adv Drug Deliv Rev. 2006; 58:212–25.

25. Burger D, Dayer JM, Palmer G, Gabay C. Is IL-1 a good therapeutic target in the treatment of arthritis? Best Pract Res Clin Rheumatol. 2006; 20:879–96.

26. Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009; 61:344–52.

27. Vignon E, Balblanc JC, Mathieu P, Louisot P, Richard M. Metalloprotease activity, phospholipase A2 activity and cytokine concentration in osteoarthritis synovial fluids. Osteoarthritis Cartilage. 1993; 1:115–20.

28. Chang DM, Chang SY, Yeh MK, Lai JH. The pharma-cokinetics of interleukin-1 receptor antagonist in Chinese subjects with rheumatoid arthritis. Pharmacol Res. 2004; 50:371–6.

29. Richette P, Francois M, Vicaut E, Fitting C, Bardin T, Corvol M, et al. A high interleukin 1 receptor antagonist/IL-1beta ratio occurs naturally in knee osteoarthritis. J Rheumatol. 2008; 35:1650–4.
30. Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADA-MTS-4 and ADAMTS-5. Arthritis Rheum. 2007; 56:575–85.

31. Lin Z, Fitzgerald JB, Xu J, Willers C, Wood D, Grodzinsky AJ, et al. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J Orthop Res. 2008; 26:1230–7.

32. Martel-Pelletier J, Pelletier JP. Osteoarthritis: A single injection of anakinra for treating knee OA? Nat Rev Rheumatol. 2009; 5:363–4.
33. Church LD, McDermott MF. Canakinumab, a fully-human mAb against IL-1beta for the potential treatment of inflammatory disorders. Curr Opin Mol Ther. 2009; 11:81–9.
Figure 1.
Experimental protocol. (1) Experiment I (6-days gel, 10 μ g of anakinra, no coacervate): upper arm. Day #0: gel preparation and anakinra loading, Day #1∼#6: daily change of media and measurement of the concentration of anakinra at the indicated days for the release pattern of anakinra, Day #4: transfer of gel into the upper compartment of the new system with plated chondrocytes on the lower compartment, and stimulation of chondrocytes with IL-1β (0.1 ng/ml) for 24 hours, Day #5: preparation of RNA and harvesting the supernatant for real time PCR and ELISA. (2) Experiment II (15-days gel, 10 or 100μ g of anakinra, coacervation with protamine): lower arm. Day #0: gel preparation and anakinra loading, Day #1∼#15: daily change of media and measurement of the concentration of anakinra at the indicated days for the release pattern of anakinra, Day #13: transfer of gel into the new system with plated chondrocytes on the lower compartment, Day #14: stimulation of chondrocytes with IL-1β (0.1 ng/ml) for 24 hours, Day #15: preparation of RNA and harvesting the supernatant for real time PCR and ELISA. IL-1Ra: IL-1 receptor antagonist, RT-PCR: real time polymerase chain reaction, ELISA: Enzyme-Linked ImmunoSorbent Assay.
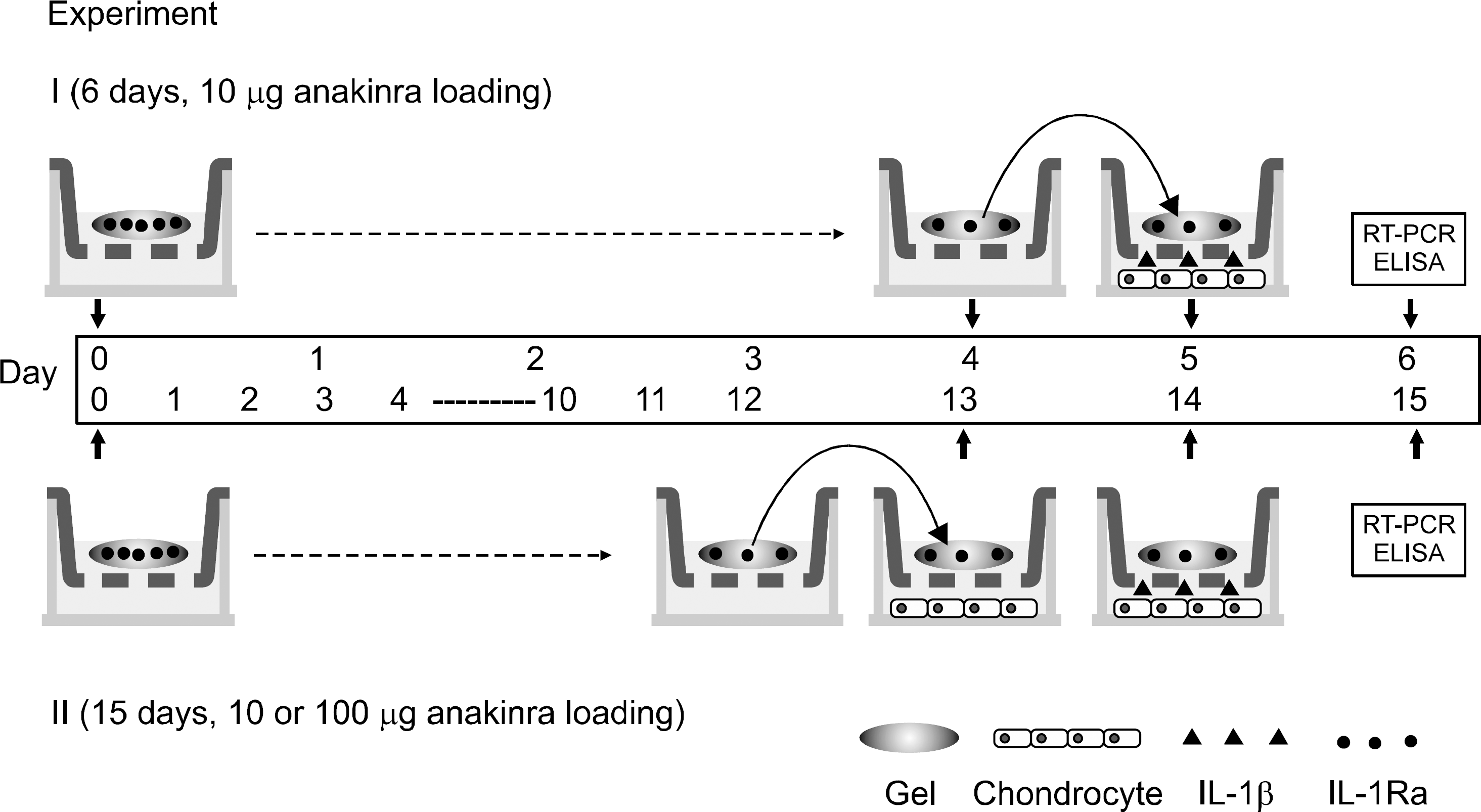
Figure 2.
Turbidity curves of protamine and the IL-1Ra mixtures. Turbidity curve at various concentration in PBS, pH 7.4, 25 o C (weight ratio of protamine to IL-1Ra: ●: 0.5, ○: 1, ▼: 2). The turbidity results indicated whether or not two macro-molecules formed the coacervate. If the turbidity was increased, then coacervate was formed. At the weight ratio of 2, the turbidity was highly increased within 3 hours. Abs: absorbance.
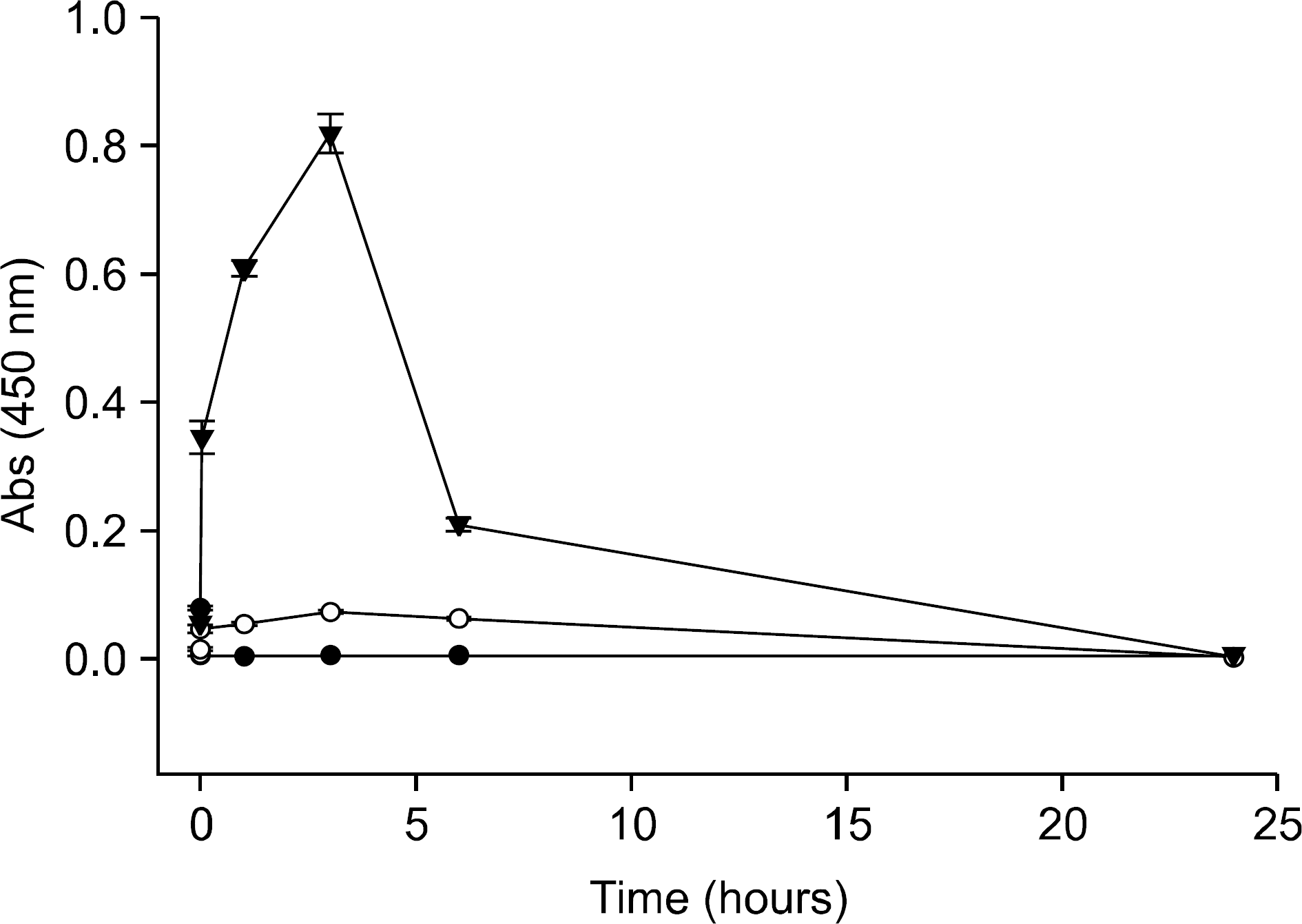
Figure 3.
In vitro release of anakinra from the thermoreversible, coacervate combination gel. The IL-1Ra released from the gel was measured by ELISA. Without coacervation with protamine, almost 80% of the loaded anakinra was released by Day #5, but coacervation made the release pattern slow down with a minimal initial burst. Only 60% and 40% of the loaded anakinra was released from the gels loaded with 10μ g and 100μ g of anakinra at Day #15, respectively. IL-1Ra: IL-1 receptor antagonist (□: 10μ g IL-1Ra, ●: 10μ g IL-1Ra+20μ g protamine, ○: 100μ g IL-1Ra+200μ g protamine).
Figure 4.
Inhibition of the gene expression of IL-1?-induced MMP-3 (Experiment I) and MMPs (Experiment II) by the anakinra released from the gel. Values are the mean and standard deviation of the indicated gene expression (duplicate measurement) relative to the untreated control. The asterisk indicates statistical significance (∗p<0.05, † p<0.001 vs. the expression level induced by IL-1β, Student's t-test). # indicates the marginal statistical significance (p=0.058 for MMP-1, p=0.059, 0.057, 0.060 respectively for MMP-13). In Experiment I, we examined the effect of the combination gel itself on the gene expression. IL-1β+ Ra indicate the stimulation with IL-1β and Ra300 without the gel in the blank bar, and the stimulation with IL-1β in the presence of the gel loaded with Ra100 in the filled bar. The gel itself (filled bar) did not influence on the result and the 6-days matured gels with anakinra suppressed the IL-1β-induced expression of MMP-3 (Experiment I). In Experiment II, the 15-days matured gels with anakinra significantly inhibited the IL-1β-induced expression of MMP-1, −2, −3 and −13 in a dose-dependent manner. MMP: matrix metalloproteinase, Ra10, 100 and 300: initial loading doses of IL-1 receptor antagonist (anakinra) at 10, 100, and 300μ g, respectively.
Figure 5.
Inhibition of the protein expression of IL-1β-induced MMP-3 in the 6-days (Experiment I) or 15-days (Experiment II) matured gel with anakinra. The values are the mean and standard deviation of the measured concentration (duplicate) of MMP-3 (ng/ml). The asterisk indicates statistical significance (∗p<0.05, † p<0.001 vs. the expression level induced by IL-1β, Student's t-test). # indicates marginal statistical significance (p=0.055 in Exp I). The gel itself (filled bar) did not have a considerable influence on the result (Experiment I). In Experiments I and II, the 6-days and 15-days matured gels with anakinra significantly inhibited the IL-1β (0.1 ng/ml)-induced expression of MMP-3 in a dose-dependent manner. The legend is same as that for Fig. 4.
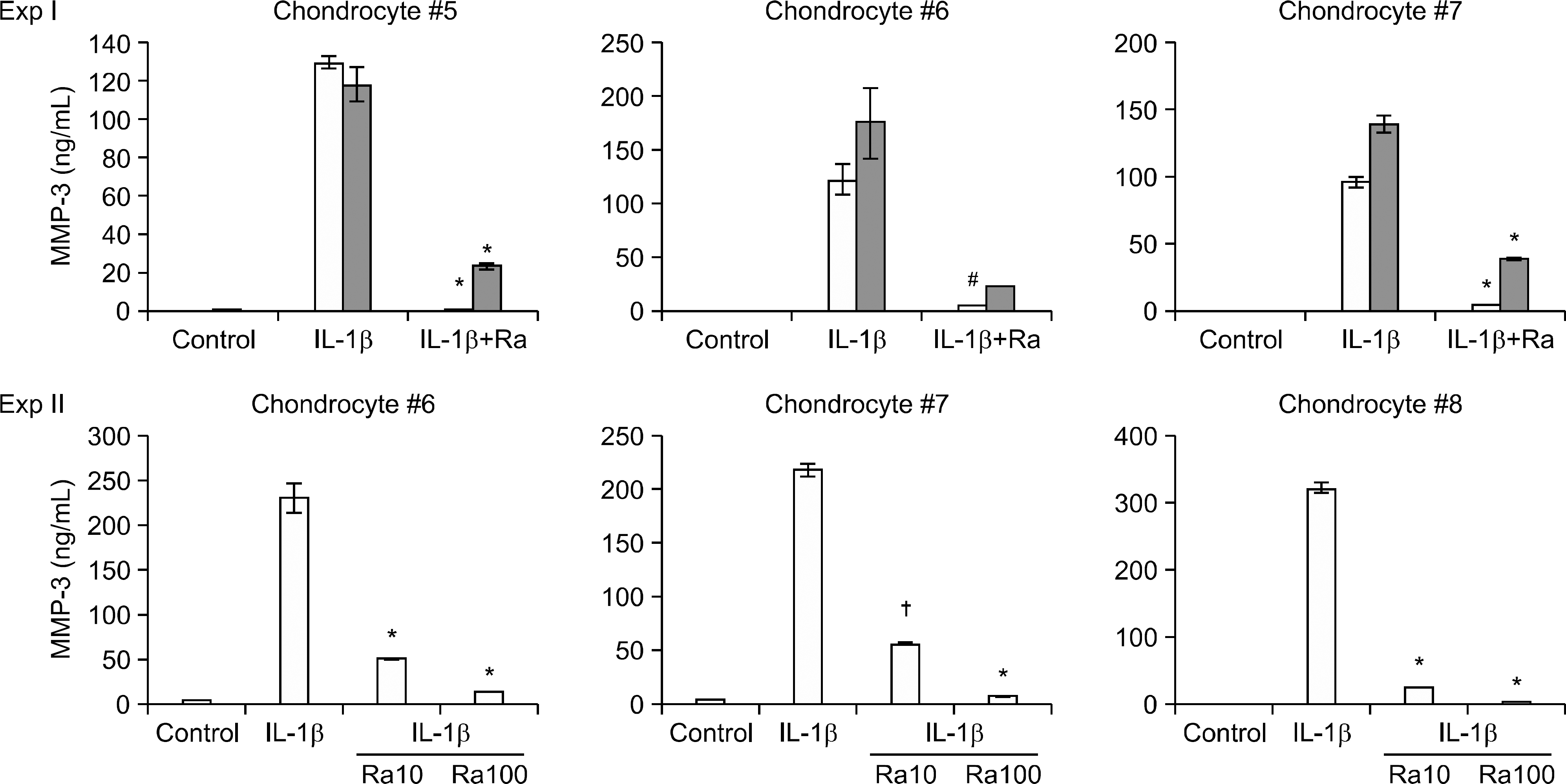
Table 1.
Primer sets used for real time PCR
Table 2.
Concentration of the released anakinra from the gels into the culture supernatant




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download