Abstract
Although parenteral nutrition (PN) is an important treatment for patients who are unable to tolerate enteral nutrition (EN), recent international guidelines recommended that PN should be reserved and initiated only after 7 days in well-nourished patients. This retrospective study was conducted to analyze the effect on clinical outcomes of a PN protocol with PN starting 4 days after admission to the intensive care unit (ICU). Eighty-one patients who were admitted from January to March of 2012 were included in the pre-protocol group, and 74 patients who were admitted from April to June of 2012 were included in the post-protocol group. There were no significant differences between the two groups when the age, gender, and admission Acute Physiology and Chronic Health Evaluation (APACHE) II scores were compared. Significantly fewer patients in the post-protocol group were provided PN (58.1% vs. 81.3%, p = 0.002), which was initiated significantly later than in the pre-protocol group (2.7 ± 2.2 days vs. 1.9 ± 2.0 days, p = 0.046). Five patients (6.2%) in the pre-protocol group acquired central line-associated bloodstream infection (CLA-BSI) in the ICU, yet none of the patients in the post-protocol group developed CLA-BSI (p = 0.03). The duration of antibiotic therapy and ICU stay were significantly shorter in the post-protocol group than in the pre-protocol group. By delaying initiation of PN, short-term clinical outcomes including incidence of CLA-BSI, antibiotic use, and ICU stay might be improved. Further research should be conducted to investigate the long-term effects of the decreased nutrient intake resulting from delayed PN.
Parenteral nutrition (PN) is an important treatment for patients unable to tolerate enteral nutrition (EN). Although PN provides life-saving nutrition, it is associated with complications such as catheter-related sepsis, metabolic abnormalities, acid-base disturbances, hyperglycemia, hypertriglyceridemia, and hepatobiliary disorders [1,2]. While systemically developed multiple international guidelines consistently recommend early initiation of EN in the intensive care unit (ICU), the guidelines do not agree on when to initiate PN in the ICU. For patients who are intolerant of or have other contraindications to EN, European guidelines recommend starting PN within 24-48 h if the patient is not expected to be on full oral nutrition within 3 days [3]. US guidelines recommended standard care (intravenous fluid) first, with PN reserved and initiated only after 7 days in well-nourished patients [4]. A randomized controlled trial (RCT) comparing early PN and late PN reported that there were fewer complications and patients were discharged from the ICU earlier in the late PN group than in the early PN group [5]. However, a total of 60% of the study patients underwent cardiac surgery, for which artificial nutrition support is rarely indicated and about half of the patients included were extubated at day 2 and discharged from the ICU at day 4, which is different from the patients in medical ICU. Heidegger et al. [6] demonstrated that patients receiving supplemental PN starting from day 4 after ICU admission experienced fewer infections and had a shorter time on mechanical ventilation than patients who did not receive supplemental PN. Despite the conflicting results of these well-designed trials, they suggest that there is no urgency to start PN. Vincent and Preiser [7] proposed that supplemental PN should not be considered before days 4-7 after ICU admission in a framework for an algorithm for PN in the ICU.
Use of the central venous catheter (CVC) to deliver total parenteral nutrition (TPN) was associated with an increased risk of central line-associated bloodstream infection (CLA-BSI), along with the length of ICU stay prior to CVC insertion and insertion in the jugular or femoral vein [8], and hyperglycemia resulting from direct infusion of glucose solutions such as TPN was related to an increased risk of infectious complications [9].
We implemented a PN protocol with PN starting 3 days after ICU admission in April, 2012. This retrospective study was conducted to analyze the effects of our PN protocol on clinical outcomes including CLA-BSI, antibiotic use, duration of ICU care, and nutritional status.
A delayed PN protocol (PN start >72 h after ICU admission) was implemented in our medical ICU beginning in April, 2012 (Figure 1). Among those patients who were admitted to the ICU from January to June of 2012, we included 155 adult patients (≥20 years of age) who stayed in the ICU longer than 3 days. Among these patients, 81 patients who were admitted from January to March of 2012 were included in the pre-protocol group, and 74 patients who were admitted from April to June of 2012 were included in the post-protocol group. Data were collected retrospectively from electronic medical records. The patients who were able to start an oral diet were excluded from the study. We collected the following data: age, gender, Acute Physiology and Chronic Health Evaluation (APACHE) II score, reasons for ICU admission, and anthropometric measures (height, weight at admission and on the 10th day of ICU admission). Daily actual calorie and protein intake from PN and EN during the first 10 days of ICU admission were calculated from the nutrition progress notes. Changes in the patients' nutritional status were evaluated by changes in prealbumin and nitrogen balance measured on the first and 10th days of ICU admission. The duration of insulin and antibiotic therapy, ICU mortality, and the length of mechanical ventilation and ICU stay were also recorded.
Data about central line-associated infectious complication episodes were provided by hospital-acquired infection controllers who were unaware of our study. CLA-BSI was defined using Center for Disease Control (CDC) criteria, i.e., a laboratory-confirmed bloodstream infection in a patient who had a central line in place for >2 calendar days on the date of the event, with the day of device placement being day 1.
The patients' nutrient requirements including energy and protein were calculated by an ICU dietitian. Energy requirements were calculated using the Harris-Benedict formula adjusted with an appropriate stress factor. The protein goal was determined by 1.2-1.8 g per kilogram of the patients' ideal body weight considering the patients' physical stress. For patients with acute or chronic kidney dysfunction, protein needs were modified considering the renal replacement therapy. For patients with no contraindications, EN was commenced as soon as hemodynamic resuscitation was accomplished. Enteral feeding was provided continuously for 18 hours a day using a feeding pump started at a lower rate, 20 mL/h, and increased gradually according to the patients' tolerance in both groups. Head-up positioning around 30° was maintained during feeding (semi-recumbent position). EN products consisted of polymeric formulas, routinely prescribed in this hospitals, containing 1.0 kcal/mL of energy (17-20% proteins, 27-45% lipids, 55-56% carbohydrates). PN was initiated after 3 days of ICU admission for the patients who failed to be provided EN. PN formulas consisted of 1.07-1.20 kcal/mL of energy (14-20% proteins, 34-35% lipids, and 45-52% carbohydrates). EN and PN formulas came from four different manufacturers and were prescribed according to each patient's estimated needs. An insulin protocol to achieve blood glucose levels ≤180 mg/dL is used in our ICU. No other treatment that might have influenced our results was introduced during either period.
This study was a retrospective one and the institutional review board waived the requirement to obtain written informed consent from the patients.
The results were expressed as mean ± SD. The differences in body mass index (BMI), the biochemical parameters (prealbumin, nitrogen balance), nutrient intake (calories and protein), and the duration of ICU stay between the two groups were analyzed with the student's t-test. A chi-square test was used to compare the differences in bloodstream infection (BSI) episodes and mortality. Statistical significance was set at p < 0.05 for all tests. The data analysis was conducted using IBM SPSS 20.0 (Armonk, NY, USA).
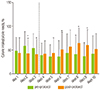
The patients were 66.0 ± 13.6 years old and 103 patients (66%) were male. The mean APACHE II score was 21.9 ± 7.6 and the primary diagnosis on ICU admission was respiratory failure (34.2%), followed by infectious (29.1%) and gastrointestinal diagnoses (7.7%), without any significant difference between the 2 groups with regard to admission diagnosis. There were no significant differences between the two groups when the age, gender, and admission APACHE II scores were compared (Table 1). Significantly fewer patients in the post-protocol group were provided PN than in the pre-protocol group (58.1% vs. 81.3%, p=0.002) (Table 2). PN was initiated significantly later in the post-protocol group than in the pre-protocol group (2.7 ± 2.2 days vs. 1.9 ± 2.0 days, p = 0.046). Although the average caloric intake via PN during the first 3 days of ICU admission was significantly lower in the post-protocol group (600 ± 393 kcal/day vs. 719 ± 330 kcal/day, p = 0.045), calorie delivery via PN during 10 days of ICU admission was not different between the two groups (453 ± 307 kcal/day vs. 458 ± 343 kcal/day, p = 0.911) (Table 2). Caloric intakes both during the first 3 days and during 10 days were similar between the two groups. Figure 2 displays the daily percentage of the calculated nutritional goal administered from day 1 through day 10 via the parenteral route. A significantly lower percentage of calories was provided in the post-protocol group on the 2nd and 3rd days of ICU admission. Caloric intake from EN plus PN during the first 10 days of ICU admission was significantly decreased after implementation of the delayed PN protocol (1,299 ± 424 kcal/day vs. 1,107 ± 480 kcal/day, p = 0.009).
Table 3 shows differences in the clinical outcomes. Although 5 patients (6.2%) in the pre-protocol group acquired CLA-BSI in the ICU, no patients in the post-protocol group developed CLABSI (p = 0.03). The duration of antibiotic therapy was significantly shorter in the post-protocol group than in the pre-protocol group (13.2 ± 9.0 days vs. 17.0 ± 11.8 days, p = 0.029). The mean stay in the ICU was shorter in the post-protocol group than in the pre-protocol group (13.4 ± 9.2 days vs. 17.1 ± 12.2 days, p = 0.037). The two groups had similar rates of death in the ICU.
After the implementation of a delayed PN protocol, the time to initiation of PN after ICU admission was prolonged, and the incidence of CLA-BSI and the use of antibiotics decreased. Although PN is an essential feeding method for patients who cannot meet their nutritional requirements through enteral nutrition, TPN is invasive and carries significant complications. One of the most serious complications of PN is catheter-related sepsis, which has been reported to occur in 1.3%-26.2% of the catheters used to administer PN [10-12]. PN has consistently been identified as an independent risk factor in the development of CLA-BSI [11], especially when the duration of PN infusion was prolonged. Once infection develops, systemic antimicrobial therapy should be given for 10-14 days or longer according to the type of organism and the severity of the infection. Therefore, CLA-BSI have been identified as a factor contributing to increasing ICU length of stay (LOS) and health care costs [13-15] and appropriate and judicious use of PN is imperative. The American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) provides guidelines for the appropriate use of PN, with the primary focus on patient selection and timing of therapy initiation and recommends that PN be initiated after 1 week, unless the patient is severely malnourished [4]. On the other hand, underfeeding has been related to negative outcomes, such as infectious complications, prolonged care in the ICU, and mortality. Delaying initiation of nutrition support exposes patients to energy deficits that cannot be compensated for later on [16], so PN might be an alternative route to provide adequate nutrients to meet increased requirements in the patients who do not tolerate EN. European guidelines emphasized the importance of adequate nutrition to prevent negative effect of calorie deficit during ICU stay [3]. We decided to delay initiation of PN only 3 days after admission to ICU, not seven days. Duration of PN infusion in the post-protocol group was significantly decreased and might be associated with significantly decreased the use of antibiotics and ICU LOS.
Many mechanisms have been proposed regarding this observation including the development of hyperglycemia during critical illness. Patients experiencing the stress of trauma, critical illness, or major surgery typically display endogenous insulin resistance that is characterized by reduced insulin uptake in peripheral tissues, along with an increase in glucose production. When combined with a large exogenous dextrose load such as occurs with TPN, the glucose oxidation capacity can easily be exceeded, which predisposes patients to develop significant hyperglycemia [17]. On the other hand, Dissanaike et al. [18] reported that maximum daily blood glucose concentrations were similar in patients with blood stream infection (BSI) and in patients without BSI. Patients with BSI received more calories parenterally than patients without BSI (36 kcal/kg/day vs. 31 kcal/kg/day, p = 0.003). Increased parenteral caloric intake is an independent risk factor for BSI in patients receiving TPN. Although calorie delivery via the parenteral route during the first 3 days of ICU admission was significantly decreased in the post-protocol group compared to the pre-protocol group, duration of insulin infusion was not significantly different between the two groups. Further studies considering the severity of hyperglycemia and prescribed dose of insulin are needed to know the underling mechanism of the relationship between CLA-BSI and PN.
A great deal of research, including the present study, has investigated relatively short-term outcomes, including complications during the ICU stay, mortality in the ICU and hospital, and 60-day mortality. In an RCT comparing standard care and early PN in critically ill adults with relative contraindications to early EN, patients receiving standard care experienced significantly greater muscle wasting and significantly greater fat loss over the duration of their ICU stays [19]. Loss of total body protein, especially skeletal muscle protein, during an ICU stay could result in increased readmission episodes, prolonged institutionalization, and compromised quality of life, which are burdens for survivors from their critical illnesses. In the current study, the patients in the post-protocol group were provided significantly less calories than those in the pre-protocol group, hence long-term outcomes should be analyzed.
As this was a retrospective study, we could not consider physicians' compliance with the protocol. Even though the number of days to initiation of PN from ICU admission was significantly increased after implementation of the protocol, it took 2.7 ± 2.2 days until PN was initiated. Although the total study period was 6 months, the possibility of difference in the medical treatment could not be excluded before and after implementing the protocol. Despite of the fact that this was a retrospective chart review, this study showed a method for practical application of the controversial international guidelines and demonstrated its effect on the risk of CLA-BSI, antibiotic use, and nutrient intake. As mentioned above, there have been different recommendations regarding the appropriate timing for initiation of PN. More practical studies should be performed according to the different patient populations, and long-term clinical outcomes as well as in-hospital outcomes should be evaluated considering the severity of hyperglycemia and prescribed dose of insulin.
In the present study, the effect of the delayed PN protocol on clinical outcomes was analyzed. After implementation of the protocol, CLA-BSI, duration of antibiotic use, and length of stay in the ICU were significantly decreased. Caloric intake during the first 10 days was significantly decreased. From the study results, we can expect positive short-term effects including lower incidence of CLA-BSI and antibiotic use, and shorter ICU stays through the delayed PN protocol. More practical studies should be performed according to the different patient populations, and long-term clinical outcomes as well as in-hospital outcomes should be evaluated. Further research should be conducted to investigate the long-term effects of the decreased nutrient intake resulting from delayed PN.
Figures and Tables
Figure 1
Delayed Parenteral Nutrition Protocol. EN: enteral nutrition, PN: parenteral nutrition, ICU: intensive care unit.

Figure 2
Percentage of daily calorie delivery via parenteral nutrition to estimated requirement for 10 days from ICU admission. *Significantly different from the pre-protocol group, p < 0.05
References
1. Btaiche IF, Khalidi N. Metabolic complications of parenteral nutrition in adults, Part 2. Am J Health Syst Pharm. 2004; 61:2050–2057.

2. Btaiche IF, Khalidi N. Metabolic complications of parenteral nutrition in adults, part 1. Am J Health Syst Pharm. 2004; 61:1938–1949.

3. Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, Griffiths R, Kreyman G, Leverve X, Pichard C. ESPEN Guidelines on parenteral nutrition: intensive care. Clin Nutr. 2009; 28:387–400.

4. McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G. A.S.P.E.N. Board of Directors. American College of Critical Care Medicine. Society of Critical Care Medicine. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: society of critical care medicine (SCCM) and american society for parenteral and enteral nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2009; 33:277–316.

5. Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, Vlasselaers D, Debaveye Y, Desmet L, Dubois J, Van Assche A, Vanderheyden S, Wilmer A, Van den Berghe G. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011; 365:506–517.

6. Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, Thibault R, Pichard C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013; 381:385–393.

7. Vincent JL, Preiser JC. When should we add parenteral to enteral nutrition? Lancet. 2013; 381:354–355.

8. van der Kooi TI, Wille JC, van Benthem BH. Catheter application, insertion vein and length of ICU stay prior to insertion affect the risk of catheter-related bloodstream infection. J Hosp Infect. 2012; 80:238–244.

9. Lin LY, Lin HC, Lee PC, Ma WY, Lin HD. Hyperglycemia correlates with outcomes in patients receiving total parenteral nutrition. Am J Med Sci. 2007; 333:261–265.

10. Beghetto MG, Victorino J, Teixeira L, de Azevedo MJ. Parenteral nutrition as a risk factor for central venous catheter-related infection. JPEN J Parenter Enteral Nutr. 2005; 29:367–373.

11. Pasero D, De Rosa FG, Rana NK, Fossati L, Davi A, Rinaldi M, Di Perri G, Ranieri VM. Candidemia after cardiac surgery in the intensive care unit: an observational study. Interact Cardiovasc Thorac Surg. 2011; 12:374–378.

12. Stratman RC, Martin CA, Rapp RP, Berger R, Magnuson B. Candidemia incidence in recipients of parenteral nutrition. Nutr Clin Pract. 2010; 25:282–289.

13. Jarvis WR. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect Control Hosp Epidemiol. 1996; 17:552–557.

14. Hu B, Tao L, Rosenthal VD, Liu K, Yun Y, Suo Y, Gao X, Li R, Su D, Wang H, Hao C, Pan W, Saunders CL. Device-associated infection rates, device use, length of stay, and mortality in intensive care units of 4 Chinese hospitals: International Nosocomial Control Consortium findings. Am J Infect Control. 2013; 41:301–306.

15. Kübler A, Duszynska W, Rosenthal VD, Fleischer M, Kaiser T, Szewczyk E, Barteczko-Grajek B. Device-associated infection rates and extra length of stay in an intensive care unit of a university hospital in Wroclaw, Poland: International Nosocomial Infection Control Consortium's (INICC) findings. J Crit Care. 2012; 27:105.e5–105.e10.

16. Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux R, Delarue J, Berger MM. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005; 24:502–509.

17. Lee H, Koh SO, Park MS. Higher dextrose delivery via TPN related to the development of hyperglycemia in non-diabetic critically ill patients. Nutr Res Pract. 2011; 5:450–454.

18. Dissanaike S, Shelton M, Warner K, O'Keefe GE. The risk for bloodstream infections is associated with increased parenteral caloric intake in patients receiving parenteral nutrition. Crit Care. 2007; 11:R114.

19. Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, Davies AR, O'Leary M, Solano T, Peake S. Early PN Investigators of the ANZICS Clinical Trials Group. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA. 2013; 309:2130–2138.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download