Abstract
This study was carried out in order to determine in vitro biocompatibility of white mineral trioxide aggregate (MTA), and to compare it with that of the commonly used materials, i. e. calcium hydroxide liner (Dycal), glass ionomer cement (GIC), and Portland cement which has a similar composition of MTA. To assess the biocompatibility of each material, cytotoxicity was examined using MG-63 cells. The degree of cytotoxicity was evaluated by scanning electron microscopy (SEM) and a colorimetric method, based on reduction of the tetrazolium salt 2,3 bis {2methoxy 4nitro 5[(sulfenylamino) carbonyl] 2H tetrazolium hydroxide} (XTT) assay.
The results of SEM revealed the cells in contact with GIC, MTA, and Portland cement at 1 and 3 days were apparently healthy. In contrast, cells in the presence of Dycal appeared rounded and detached. In XTT assay, the cellular activities of the cells incubated with all the test materials except Dycal were similar, which corresponded with the SEM observation. The present study supports the view that MTA is a very biocompatible root perforation repair material. It also suggests that cellular response of Portland cement and GIC are very similar to that of MTA.
In endodontic practice, procedural accident such as root perforation may occur and affect the prognosis of root canal treatment. Root perforation often leads to treatment failure. A nonsurgical approach is recommended as the first choice for the treatment of root perforation1). Root perforation repair materials must be biocompatible to minimize adverse effects on periodontal tissues and alveolar bone induced by direct contact2). Some materials have been suggested for this purpose including calcium hydroxide containing material (ex. Dycal), glass-ionomer cement and mineral trioxide aggregate (MTA).
Calcium hydroxide has been established for the last 3 decades as the most popular material for restricting bacterial contamination and providing the appropriate chemical stimulation for tissue healing in various endodontic situations where formation of calcific barriers are necessary. It is the commonly used dental material for direct pulp capping, pulpotomy, apical closure, root fractures, or treatment of root resorption. It has also been introduced as a perforation repair material3).
Glass ionomer cements (GIC), since their introduction, have undergone considerable modification in formulation and have been endorsed for use in a number of clinical applications4). GIC has been advocated for use as a perforation repair material1). The advantage of GIC over other materials is its adhesiveness to dentine, and several studies, in vitro, demonstrate its good sealing ability1). Preliminary data suggested that the cytotoxicity of GIC was very low5).
Mineral trioxide aggregate (MTA) is an endodontic material that was first used as a root-end-filling material6), but it has also been used as an alternative in several clinical procedures, such as capping of pulp tissue, root-end closure, for repair of furcal perforations and forming an apical barrier7,8). When MTA is used as a perforation repair material, it directly contacts fibroblasts, cementoblasts, and osteoblastic cells of the periodontal ligament. Therefore the cellular response to MTA is important for the repair and regeneration of periradicular tissues9).
Recently, there has been great interest in the evaluation of Portland cement as an alternative to MTA8,10). Many studies compared MTA with Portland cement and indicated that they had a similar chemical composition and biocompatibility11,12).
The purpose of this study was to compare the biocompatibility MTA to that of other root perforation repair materials, such as calcium hydroxide liner (Dycal), glass ionomer cement (GIC) and Portland cement by examining cytotoxicity through scanning electron microscope (SEM)and A colorimetric method, based on reduction of the tetrazolium salt 2,3bis{2methoxy 4nitro 5[(sulfenylamino) carbonyl] 2H tetrazolium hydroxide}(XTT) assay in MG-63 cells.
The selected cell line, MG-63 cells (Korean Cell Line Bank, Korea) derived from human osteosarcoma, were grown in Dulbeco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics in a humidified 37℃ CO2 incubator.
Four materials were tested: calcium hydroxide liner (Dycal®,Dentsply Caulk, Milford, USA), glass ionomer cement (GIC: Fuji II GC, Tokyo, Japan), white mineral trioxide aggregate (MTA: ProRoot® MTA, Dentsply Tulsa Dental, Tulsa, OK, USA), and white Portland cement (PC: White Portland Cement® Union White Cement industrial Co. LTD., Seoul, Korea). Dycal, GIC, and MTA were mixed according to the manufacturer's instructions. Portland cement was sterilized with ethylene oxide and mixed to a consistency similar to MTA. The samples were fabricated in a sterile cylindrical polyethylene tube, and yielded the discs of the size of 5 mm in diameter and 3 mm in height. The materials were mixed and placed into the tube. Excess flash was removed. After the samples had set, they were sterilized with ethylene oxide.
Samples of test material (2 samples for each material) were placed in the bottom of 96 well culture plates with one disc per well and immersed in DMEM for 72 hours. After this period, the media was discarded. MG-63 cells were seeded into the wells at 3 × 105 cells per well with 300 µl medium. The plates were then incubated for 1 day and 3 days. After incubation, the discs of materials along with the cells grown on their surface were washed three times with tris-buffered saline (TBS) for 10 minutes each, fixed with 2.5% glutaraldehyde in 0.1 M TBS buffer (pH 7.4) for 2 hours and rinsed with TBS solution. The samples were dehydrated in ascending grades of ethanol (40% for 10 minutes, 50% for 10 minutes, 60% for 10 minutes, 70% for 10 minutes, 80% for 10 minutes, 90% for 10 minutes, and three times in 100% for 10 minutes each), dried for 24 hours in 37℃ CO2 incubator, and sputter-coated with 15 nm gold palladium. All solutions were filtered for sterilization. The discs were examined by a scanning electron microscope (Hitachi S4700, Tokyo, Japan) at various magnifications.
The effect of the extract media on MG-63 cells was evaluated by XTT assay. The extracts were prepared in a similar manner to the method reported elsewhere13,14). Describing briefly, 6 sample discs per material were placed into each well of a 48 well culture plate and 1ml DMEM without fetal bovine serum, to prevent any bacterial growth and with 1% antibiotics was added. The plate was maintained on a roller mixer at 4℃.
Extracts of 1, 4, and 7 days were obtained and treated with same volume of DMEM supplemented with 20% fetal bovine serum and 1% antibiotics to make 10% fetal bovine serum. The extracts were filtered for sterilization.
This assay is based on the cleavage of the tetrazolium salt into the water-soluble formazan by succinate-tetrazolium reductase system which belongs to the respiratory chain of the mitochondria and active only in the viable cells. Therefore, the amount of the formazan dye is directly proportional to the number of living cells. The cells were placed in 96 well plates at a density of 1.0 × 105 cells per well and allowed to attach for 24 hours. After overnight attachment, each well was treated with 100 µl of the material extracts. One hundred microliter of DMEM supplemented with 10% fetal bovine serum and 1% antibiotics was used as the negative control. One hundred microliter of DMEM supplemented with 10% fetal bovine serum and 1% antibiotics with cells was used as the positive control. The cells were exposed to the extracts obtained after 1, 4, and 7 days from test material incubations, and XTT assay was performed with the cells after exposing 1, 3, and 8 days. XTT reagent was added to the well, 10 µl per well, and the wells were incubated for 30 minutes. The UV absorbances of each test well were measured in a multiwell spectrophotometer (ELX 800UV, Bio-Tek Instrument, Inc.) at primary wave length of 450 nm and reference wave length of 630 nm.
Wilcoxon Signed Rank Test was used to determine statistical significance of observed differences according to the cultivation time. One way analysis of variance followed by Bonferroni test was used to compare cytotoxicity by day of extracts and test materials. Statistical significance was assigned when p<0.05.
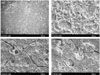
Both 1 day and 3 days, in Dycal, a few rounded cells were observed on the material, but no living cells were seen. In contrast, the MTA, GIC and Portland cement showed flattened cells in close proximity to one another, and these were seen to be spreading across the substrate. Numerous thin cytoplasmic extensions were also observed, and they were projected from the cell to the surrounding surface or adjacent cells.
Figure 1 shows the surface of cells in the presence of test discs after the incubations of 1 day. The findings were similar in all the specimens regardless of the duration of the contact incubations.
Figure 2 shows the calculated relative cell viability (absorbance of test material / Absorbance of (+)control × 100 (%)) of MG-63 cells after being exposed to the extracts obtained from the culture wells.
By accessing the cell viability of MG-63 cells, in all extracts, GIC, MTA and Portland cement had a similar effect on MG-63 cells. They exhibited higher cell viability for up to 8 days. And that was in close proximity to the control.
After 1 day, cell viability of Dycal was significantly decreased, and most of cells had died within 1 day. There was no difference according to the extracts of different extraction time. There were no significant differences among cultivation time in the each test material. Comparing 1, 4, and 7 days extracts of each material, there were no certain tendency.
In vitro methods have been recommended for evaluating the cytotoxicity of endodontic materials and several model systems, using established cell lines as well as primary cells, have become available for screening purposes2). The human osteosarcoma cell line (MG-63) was chosen for this experiment because of availability and ease of culture. The cell line's response with respect to cytokine production has been shown to be similar to that of human osteoblasts16).
Many researchers have employed the use of different cell-material contact methods for evaluating the cytotoxicity. Cell-material contact may be direct, indirect or through an eluate15). In this study, scanning electron microscope finding was done as cell-material direct contact method, and XTT assay was done through an eluate.
The use of scanning electron microscope to examine the cytomorphology of established cell lines in the presence of materials as ways of assessing cytotoxicity have been widely used16). Adhesion and spreading of cells on a material surface are the initial phase for cellular function. The persistence of rounded cells with little or no spreading suggest the surface material may be toxic. Cell adhesion and spreading on materials could be used as a criterion for evaluation of materials17). This study showed the early cellular response of MG-63 cells by observation of cell morphology on surface of materials using scanning electron microscope.
In this study, MG-63 cells were attached, spread, and proliferated to form a matrix-like layer on the surface of GIC, MTA, and Portland cement. A few rounded cells were observed on Dycal, but no living cells were seen. These results are in good agreement with previous reports9,10,16,18-20).
Previous studies have shown that the processing method used for SEM caused precipitation of calcium carbonate polymorphs, resulting in artifacts and difficulty in viewing cellular morphology. The phosphate in the fixing buffer reacts with the calcium hydroxide producing calcium phosphate crystals that could be identified under the scanning electron microscope (SEM)21,22). In view of this, phosphate buffered saline (PBS) was avoided in fixing buffer and substituted with tris-buffered saline (TBS) to avoid precipitation of calcium phosphate crystals.
A colorimetric method based on the tetrazolium salt, XTT, was first described by Scudiero et al23) in 1988. Whilst the use of MTT produced a non-soluble formazan compound which necessitated dissolving the dye in order to measure it, the use of XTT produces a soluble dye. The use of XTT greatly simplifies the procedure of measuring proliferation, and is, therefore, an excellent solution to the quantitating of cells and their viability without using radioactive isotopes.
When cell viability is tested, extract medium from test material was used to prevent its direct contact with cells, because Camilleri et al13) reported that direct contact between cements and cells inhibited cell growth.
The results of this study showed that GIC, MTA, and Portland cement had no cytotoxic effect compared with the control. In contrast, Dycal exhibited strong cytotoxicity. The cytotoxicity of calcium hydroxide in pulp cells and fibroblst was also investigated as a pulp capping material or a root canal sealer10,24,25). Many studies indicated that it caused cytolysis because of it's high alkaline property24). Although in this study different cell line, MG-63 cells were used, Dycal showed cytotoxicity.
In this study, the result showed similarly high cell viability of both MTA and Portland cement. Recently, findings of studies where MTA was compared with Portland cement have shown that these two materials appear to be almost identical10). The biocompatibility of Portland cement has previously been documented26). In this study, we attempted to evaluate the biologic effects of Portland Cement on cultured osteosarcoma cells by means of a cell viability test, and SEM observation.
There was not any apparent difference according to the length of time of extraction in all materials. It is considered that this was because of conditions for preparing the test material and in the aging time. All test cements were aged over the 3 days and set almost completely. Therefore, it is considered that there were no particular difference between the composition of toxic material of extract day. Further studies will be needed to compare the cytotoxicity of early stage after setting of test material to that of the later stage.
In this study we compared to the biocompatibility of MTA to that of biocompatibility of other root perforation repair materials, such as calcium hydroxide liner (Dycal), glass ionomer cement (GIC) and Portland cement by examining cytotoxicity through the scanning electron microscope (SEM) and XTT assay through extract in MG-63 cells from human osteosarcoma cells.
The SEM revealed the cells in contact with GIC, MTA, and Portland cement at 1 and 3 days were apparently healthy. In contrast, cells in the presence of Dycal appeared rounded and detached. In XTT assay, the cellular activities of the cells incubated with all the test materials except Dycal were similar, which corresponded with the SEM observation. There were no significant differences according to the incubation times. There was no certain tendency, according to the length of time of extraction.
The present study supports the view that MTA is a very biocompatible root perforation repair material. Also It suggests that cellular response of Portland cement and GIC are very similar to MTA. And also, it suggests the possibility of using modified Portland cement or modified GIC as a inexpensive substitute for MTA in endodontics. But further studies will be needed to compare cytotoxicity of the early stage after setting of test material to the cytotoxicity of later stage.
Figures and Tables
References
1. Vajrabhaya LO, Korsuwannawong S, Jantarat J, Korre S. Biocompatibility of furcal perforation repair material using cell culture technique: Ketac Molar versus ProRoot MTA. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006. 102:e48–e50.

2. Souza NJ, Justo GZ, Oliveira CR, Haun M, Bincoletto C. Cytotoxicity of materials used in perforation repair tested using the V79 fibroblast cell line and the granulocyte-macrophage progenitor cells. Int Endod J. 2006. 39:40–47.

3. Tziafas D, Economides N. Formation of crystals on the surface of calcium hydroxide-containing materials In vitro. J Endod. 1999. 25:539–542.

4. Makkawy HA, Koka S, Lavin MT, Ewoldsen NO. Cytotoxicity of root perforation repair materials. J Endod. 1998. 24:477–479.

5. de Souza Costa CA, Hebling J, Garcia-Godoy F, Hanks CT. In vitro cytotoxicity of glass-ionomer cements. Biomaterials. 2003. 24:3853–3858.
6. Yun YR, Yang IS, Hwang YC, Hwang IN, Choi HR, Yoon SJ, Kim SH, Oh WM. Pulp response of Mineral trioxide aggregate, calcium sulfate or calcium hydroxide. J Korean Acad Conserv Dent. 2007. 32:95–101.

7. Gorduysus M, Avcu N, Gorduysus O, Pekel A, Baran Y, Avcu F, Ural AU. Cytotoxic effects of four different endodontic materials in human periodontal ligament fibroblasts. J Endod. 2007. 33:1450–1454.

8. Chang SW, Yoo HM, Park DS, Oh TS, Bae KS. Ingredients and cytotoxicity of MTA and 3 kinds of Portland cements. J Korean Acad Conserv Dent. 2008. 33:369–376.

9. AL-Rabeah E, Perinpanayagam H, MacFarland D. Human alveolar bone cells interact with ProRoot and tooth-colored MTA. J Endod. 2006. 32:872–875.

10. Min KS, Kim HI, Park HJ, Pi SH, Hong CU, Kim EC. Human pulp cells response to Portland cement in Vitro. J Endod. 2007. 33:163–166.

11. Funteas UR, Wallace JA, Fochtman EW. A comparative analysis of mineral trioxide aggregate and Portland cement. Aust Endod J. 2003. 29:43–44.

12. Saidon J, He J, Zhu Q, Safavi K, Spångberg LS. Cell and tissue reactions to mineral trioxide aggregate and Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003. 95:483–489.

13. Camilleri J, Montesin FE, Di Silvio L, Pitt Ford TR. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J. 2005. 38:834–842.

14. Kim HJ, Baek SH, Bae KS. Cytotoxicity and genotoxicity of newly developed calcium phosphate-based root canal sealers. J Korean Acad Conserv Dent. 2006. 31:36–49.

15. Saw TY, Cao T, Yap AU, Lee Ng MM. Tooth slice organ culture and established cell line culture models for cytotoxicity assessment of dental materials. Toxicol in Vitro. 2005. 19:145–154.

16. Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to Mineral Trioxide Aggregate. J Endod. 1998. 24:543–547.

17. Zhu Q, Haglund R, Safavi KE, Spangberg LS. Adhesion of human osteoblasts on root-end filling materials. J Endod. 2000. 26:404–406.

18. Mitchell PJ, Pitt Ford TR, Torabinejad M, McDonald F. Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials. 1999. 20:167–173.

19. Balto HA. Attachment and morphological behavior of human periodontal ligament fibroblasts to mineral trioxide aggregate: a scanning electron microscope study. J Endod. 2004. 30:25–29.

20. Torabinejad M, Hong CU, Pitt Ford TR, Kettering JD. Cytotoxicity of four root end filling materials. J Endod. 1995. 21:489–492.

21. Camilleri J, Montesin FE, Papaioannou S, McDonald F, Pitt Ford TR. Biocompatibility of two commercial forms of mineral trioxide aggregate. Int Endod J. 2004. 37:699–704.

22. Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TR. The constitution of mineral trioxide aggregate. Dent Mater. 2005. 21:297–303.

23. Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988. 48:4827–4833.
24. Leonardo RT, Consolaro A, Carlos IZ, Leonardo MR. Evaluation of cell culture cytotoxicity of five root canal sealers. J Endod. 2000. 26:328–330.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download