Abstract
Diabetes Mellitus (DM) is a syndrome accompanied with the abnormal secretion or function of insulin, a hormone that plays a vital role in controlling the blood glucose level (BGL). Type 1and 2 DM are most common form and the prevalence of the latter is recently increasing. The aim of this article was to assess whether Type 2 DM could act as a predisposing risk factor on the pulpo-periapical pathogenesis. Previous literature on the pathologic changes of blood vessels in DM was thoroughly reviewed. Furthermore, a histopathologic analysis of artificially-induced periapical specimens obtained from Type 2 diabetic and DM-resistant rats was compared. Histopathologic results demonstrate that the size of periapical bone destruction was larger and the degree of pulpal inflammation was more severe in diabetic rats, indicating that Type 2 DM itself can be a predisposing risk factor that makes the host more susceptible to pulpal infection. The possible reasons may be that in diabetic state the lumen of pulpal blood vessels are thickened by atheromatous deposits, and microcirculation is hindered. The function of polymorphonuclear leukocyte is also impaired and the migration of immune cells is blocked, leading to increased chance of pulpal infection. Also, lack of collateral circulation of pulpal blood vessels makes the pulp more susceptible to infection. These decrease the regeneration capacity of pulpal cells or tissues, delaying the healing process. Therefore, when restorative treatment is needed in Type 2 DM patients, dentists should minimize irritation to the pulpal tissue un der control of BGL.
Figures and Tables
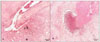 | Figure 2Histopathological analysis of block specimens including mandibular first molars and periapical bone. In the OLEFT (A, Type 2 DM rat) group, there were lots of inflammatory cells such like macrophages, lymphocytes, and plasma cells in the large periapical lesion of the mesio-buccal roots (A). In the LETO (B, DM-resistant rat) group, there was relatively small apical lesion with inflammatory cells and apoptotic bodies(×100, unpublished data). |
References
1. Diabetes. 2000. 09. 04. Newsweek.
2. Bender IB. Diabetes and the dental pulp. J Endod. 2003. 29:383–389.
3. Soskolne WA, Klinger A. The relationship between periodontal diseases and diabetes: an overview. Ann Periodontol. 2001. 6:91–98.

4. Ilgüy M, et al. Dental lesions in adult diabetic patients. N Y State Dent J. 2007. 73:58–60.
5. Mealey BL. Impact of advances in diabetes care on dental treatment of the diabetic patient. Compend Contin Educ Dent. 1998. 19:41–58.
6. Campbell J, et al. Pancreatic islet ultrastructure, serum and pancreatic immunoreactive insulin in somatotrophic and mestasomatrophic diabetes in dogs. J Submicrosc Cytol. 1981. 13:599–608.
7. Yoon JW. The role of viruses and environmental factors in the induction of diabetes. Curr Top Microbiol Immunol. 1990. 164:95–123.

8. Witko-Sarsat V, Deschamps-Latscha B. Neutrophil -derived oxidants and proteinases as immuno-modulating mediators in inflammation. Mediators Inflamm. 1994. 3:257–273.

9. Schmid-Schonbein GW, et al. Meiselman HJ, Liehtman MA, La Celle PL, editors. Viskoelastic deformation of white cells. Theory and analysis. White cell mechanics: basic science and clinical aspects. 1984. New York: Alan R. Liss, Inc.;19–51.
10. Krell V, et al. Light and electron microscopic findings. J Endod. 1994. 20:469–473.
11. Vlassara H. Recent progress in advanced glycation end products and diabetic complications. Diabetes. 1997. 46:Suppl 2. S19–S25.

12. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular disease. the role of oxidant stress. Circ Res. 2000. 87:840–844.

13. Sen CK, Packer L. Antixoidant and redox regulation of gene transcription. FASEB J. 1996. 10:709–720.
14. Matsubara T, Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986. 137:3295–3298.
15. Li PF, et al. Reactive oxygen species induce apoptosis of vascular smooth muscle cell. FEBS Lett. 1997. 404:249–252.

16. Griendling KK, et al. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994. 74:1141–1148.

17. Zalba G, et al. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension. 2000. 35:1055–1061.

18. Chun KJ, et al. Mechanism of Impaired Endothelium-dependent Vasodilation in Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. J Korean Diabetes Assoc. 2002. 26:46–56.
19. Iijima R, et al. Novel biological function of sialic acid(N-acetylneuraminic acid) as a hydrogen peroxide scavenger. FEBS Lett. 2004. 561:163–166.

20. Iijima R, et al. Characterization of the reaction between sialic acid(N-acetylneuraminic acid) and hydrogen peroxide. Biol Pharm Bull. 2007. 30:580–582.

22. Bissada NF, Sharawy AM. Histologic study of gingival and pulpal vascular changes in human diabetics. Egypt Dent J. 1970. 16:283–296.
23. Fouad AF. Diabetes mellitus as a modulating factor of endodontic infections. J Dent Educ. 2003. 67:459–467.

24. Suchitra U, et al. In search of endodontic pathogens. Kathmandu Univ Med J (KUMJ). 2006. 4:525–529.
25. Robinson HBG, Boling LR. Anochoretic effect in pulpitis. J Am Dent Assoc. 1968. 28:268.
27. Kawashima N, et al. Effect of NOS inhibitor on cytokine and COX2 expression in rat pulpitis. J Dent Res. 2005. 84:762–767.

28. Deguchi S, et al. role of lipopolysaccharide. J Periodontal Res. 1990. 25:293–299.
29. Bhoola KD, et al. kallikreins, kininogens, and kininases. Pharmacol Rev. 1992. 44:1–80.
30. Tiffany CW, Burch RM. Bradykinin stimulates tumor necrosis factor and interleukin-1 released from macrophages. FEBS Lett. 1989. 247:189–192.

31. Bhoola KD. Translocation of the neutrophil kinin moiety and changes in the regulation of kinin receptors in inflammation. Immunopharmacology. 1996. 33:247–256.

33. Vlassara H. Recent progress in advanced glycation end products and diabetic complications. Diabetes. 1997. 46:S19–S25.

34. Fouad AF, et al. PCR-based identification of bacteria associated with endodontic infections. J Clin Microbiol. 2002. 40:3223–3231.

35. Kum KY, Fouad AF. PCR-based identification of Eubacterium spp. and Eggerthella denta in endodontic infection. J Korean Acad Conserv Dent. 2003. 28:241–248.

36. Fouad AF, Kum KY, Zhu Q. Molecular characteristics of the presence of Eubacterium spp. and Streptococcus spp. in endodontic infections. Oral Microbiol Immunol. 2003. 18:249–255.

37. Sbordone L, et al. Periodontal status and selected cultivable anaerobic microflora of insulin-dependent juvenile diabetics. J Periodontol. 1995. 66:452–461.

38. Sbordone L, et al. a 3-year longitudinal study. J Periodontol. 1998. 69:120–128.
39. Ay S, et al. Assessment of mandibular bone mineral density in patients with Type 2 diabetes mellitus. Dentomaxillofac Radiol. 2005. 34:327–331.

40. Kohsaka T, et al. Periapical lesions in rats with streptozotocin-induced diabetes. J Endod. 1996. 22:418–421.

41. Iwama A, et al. The effect of high sugar intake on the development of periradicular lesions in rats with Type 2 diabetes. J Dent Res. 2003. 82:322–325.

42. Ward DT, et al. Functional, molecular, and biochemical characterization of streptozotocin-induced diabetes. J Am Soc Nephrol. 2001. 12:779–790.

43. Schneider L, Schedl H. Diabetes and intestinal calcium absorption in rat. Am J Physiol. 1972. 223:1319–1323.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download