Abstract
In this study, we investigated the in vitro antioxidative effects, antimicrobial activities and single oral dose toxicity of the extracts from Samwhang-sasimtang to evaluate its use as a functional ingredient in cosmetics. In the antioxidative effect, the ethanol extract from Samwhang-sasimtang (SSE) had higher antioxidant values of 91.9% at 1,000 µg/mL than that of water extract from Samwhang-sasimtang (SSW, 77.0%) when evaluated by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity. 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation decolorization of SSE was 82.2%, higher than that of the SSW (55.0%) and the antioxidant protection factors (APF) of SSW and SSE were 1.64 and 1.62 at 1,000 µg/mL in concentration, respectively. This study was also undertaken to test the in vitro antimicrobial activity with the extracts of Samwhang-sasimtang. In general, the SSE showed the significant antimicrobial activity against Staphylococcus aureus and Staphylococcus epidermidis. In single oral dose toxicity study, there were no differences in vivo were observed between control and treated groups in clinical signs, body weight gains, and gross finding. The results indicated that SSE did not show any toxic effects at 10 mL/kg in mice, and the LD50 of SSE was found to be higher than 10 mL/kg in this experiment. In conclusion, the extracts from Samwhang-sasimtang may act as a natural subsistence for functional cosmetics.
REFERENCES
Andarwulan N.., Shetty K.1999. Phenolic content in differentiated tissue cultures of untransformed and Agrobacterium transformed roots of anise (Pimpinella anisum L.). J. Agric. Food Chem. 47(4):1776–1780.
Blois M.S.1958. Antioxidant determinaion by the use of stable free radical. Nature. 81:1198–1199.
Pellegrin N.., Roberta K.., Min Y.., Catherine R.E.1999. Screening of dietary carotenoid and carotenoid-rich fruit extracts for antioxidant activities applying 2,2'-azino-bis(3-ethylenebenzothiazoline-6-sulfonic acid) radical cation decolorization assay. In Method in Enzymology, 299, pp.379–389. Academic Press, New York and London.
Ham S.S.., Oh D.H.., Hong J.K.., Lee J.H.1997. Antimutagenic effects of juices from edible Korean wild herbs. J. Food Sci. Nutr. 2(2):155–161.
Higashi G.S.2000. Appraisement of antioxidative activity from vegetables. Jpn. J. Food Ind. 57(1):56–64.
Jeong J.H.., Wee J.J.., Shin J.Y.., Cho J.H.., Jung D.H.2005. Antioxidative effect of crude saponin fraction prepared from culture product of basidiomycota cultured with fresh ginseng as substrate. Kor. J. Food Sci. Technol. 37(1):67–72.
Kim H.J.., Lim H.W.., Choi S.W.., Yoon C.S.2006. Antimicrobial effect of ethanol extract of Dryopteris crassirhizoma nakai on Propionibacterium acnes. J. Soc. Cosmet. Scientists Kor. 32(3):201–208.
Masaki H.., Sakaki S.., Atsumi T.., Sakurai H.1995. Active-oxygen scavenging activity of plants extracts. Biol. Pharm. Bull. 18(1):162–166.
Oatsuka K.1963. Practical treatment of herbal medicine for various symptoms, pp. 17, 453, 653, 679, Nanjantou, Tokyo, Japan.
Oh M.H.., Whang H.J.2003. Chemical composition of server herb plants. Kor. J. Food Sci. Techol. 35(1):1–6.
Ryoo J.W.., Cha B.C.1998. Mineral content and antioxidative activity in some herb plants. Kor. J. Med. Crop. Sci. 6(1):28–32.
Shin H.J.2007. A trend in research and development of natural gardenia pigments. Kor. J. Biotechnol. Bioeng. 22(5):271.
Suzuki Y.., Inoue T.., Fukuda H.1991. The Japenese pharmacopoeia, 12th ed., pp. D-116, D-132, D-576, Hirokawa Shyoudden, Tokyo, Japan.
Torel J.., Gillard J.., Gillard P.1986. Antioxidant activity of flavonoids and reactivity with peroxy radical. Phytochemistry. 25:383–385.

Wiseman H.1996. Dietary influences on membrane function: Important in protection against oxidative damage and disease. J. Nutr. Biochem. 7(1):2–6.
Figure 1.
Antioxidative effect of Samwhang-sasimtang extracts on DPPH radical scavenging activity. SSW, Samwhang-sasimtang water extracts; SSE, Samwhang-sasimtang ethanol extracts. Data shown are mean values with SD (n=3). The value of each group statistically significant as compared with control (∗P<0.05).
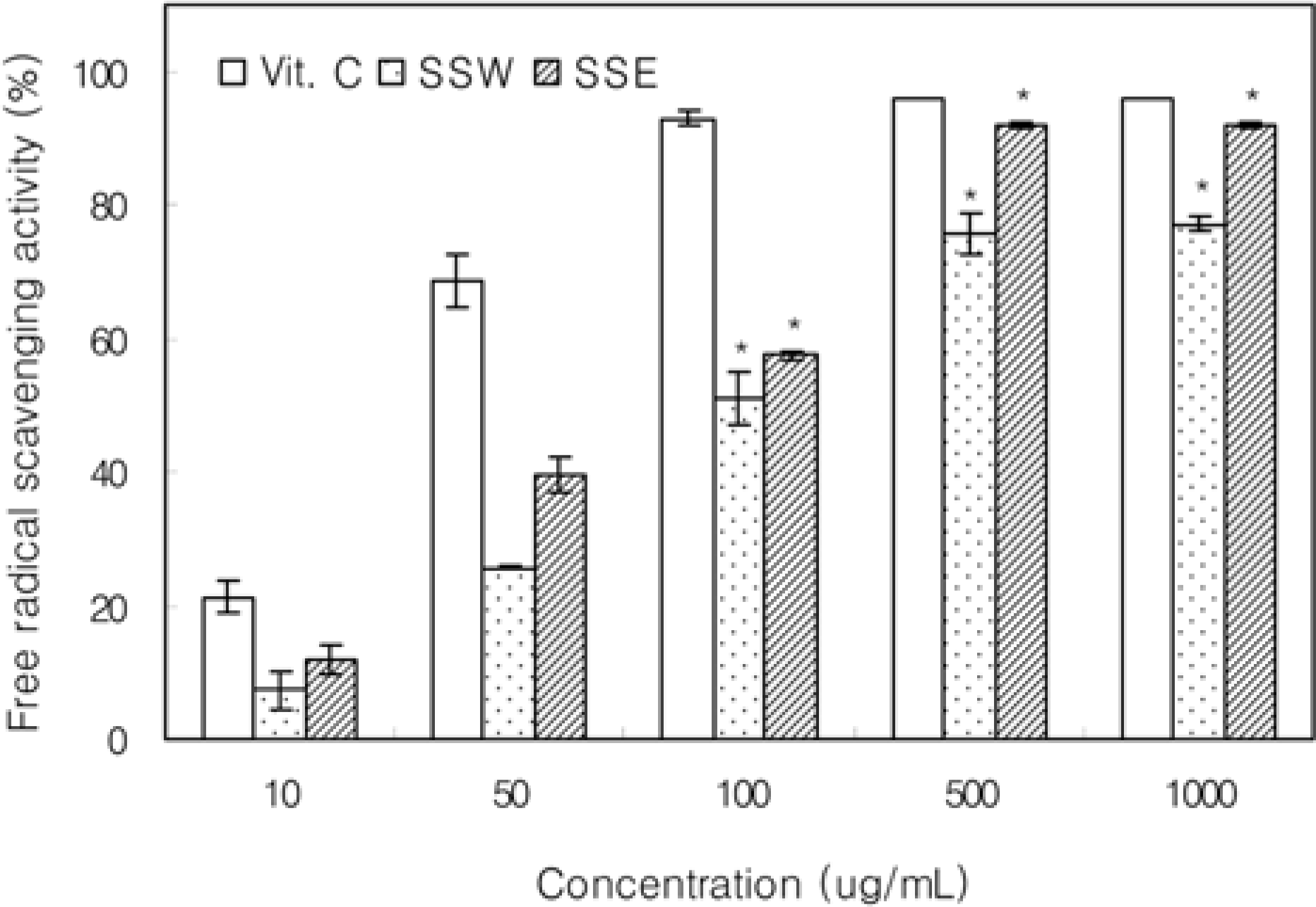
Figure 2.
Antioxidative effect of Samwhang-sasimtang extracts measured by ABTS radical cation decolorization. SSW, Samwhang-sasimtang water extracts; SSE, Samwhang-sasimtang ethanol extracts. Data shown are mean values with SD (n=3). The value of each group statistically significant as compared with control (∗P<0.05).
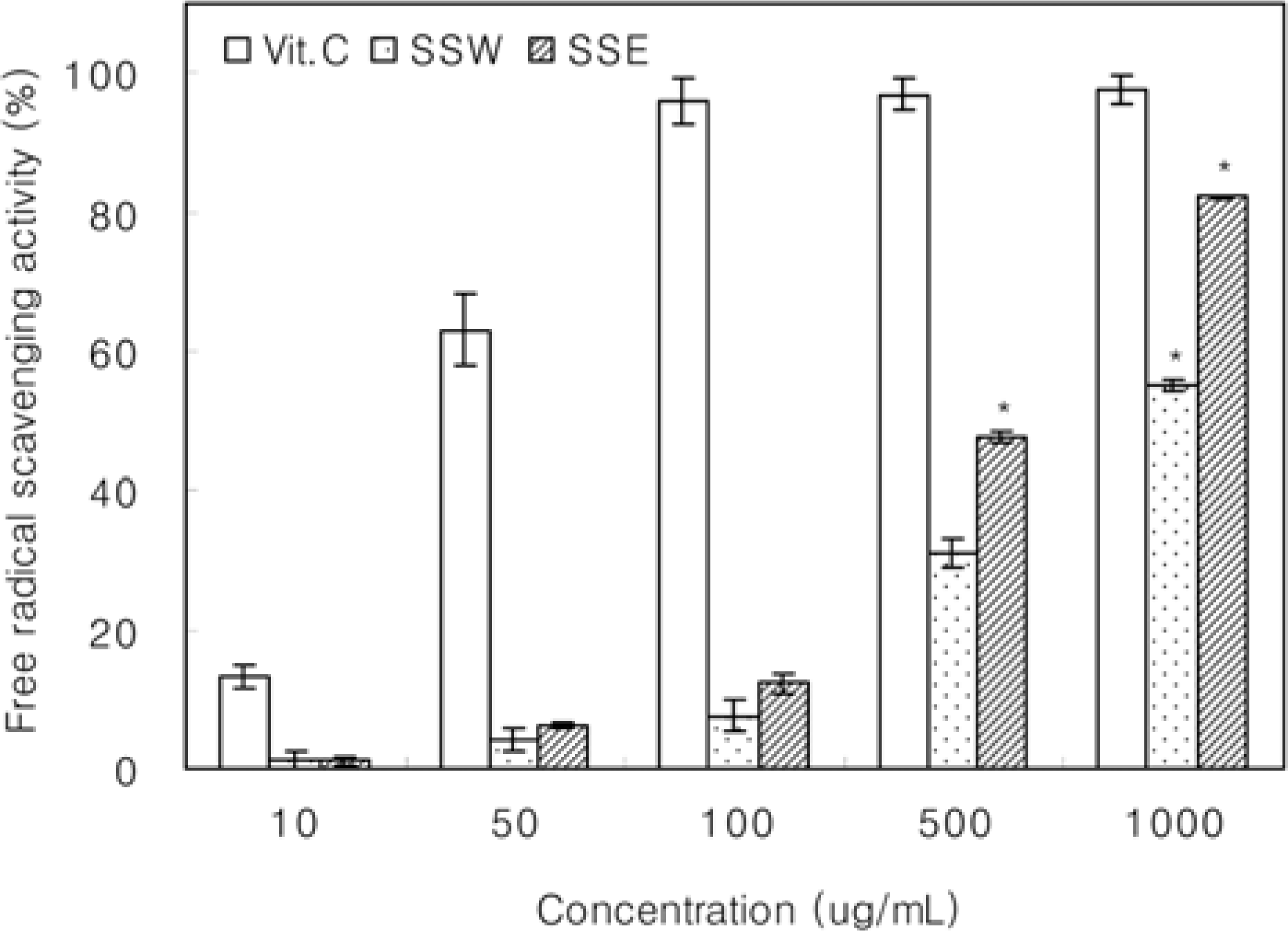
Table 1.
Antioxidative effect of Samwhang-sasimtang extracts by antioxidant protection factor
Table 2.
Antimicrobial activities of Samwhang-sasimtang extracts against several microorganisms
| Strains | Concentration (mg/8 mm paper disc) | Clear zone on plate (mm)1 | |
|---|---|---|---|
| SSW | SSE | ||
| 0.5 | -2 | - | |
| Staphylococcus aureus | 1 | - | 9.0 |
| 2 | - | 12.0 | |
| 4 | - | 16.3 | |
| 0.5 | - | - | |
| Staphylococcus epidermidis | 1 | - | 9.0 |
| 2 | - | 10.0 | |
| 4 | - | 11.0 | |
| 0.5 | - | - | |
| 1 | - | - | |
| Escherichia coli | 2 | - | - |
| 4 | - | - | |
Table 3.
Mortality of male and female mice orally treated with Samwhaing-sasimtang ethanol extract (1%)
| Material | Sex | Injected volume (mL/kg) | Hours after treatment | Days after treatment | Final mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 3 | 7 | 14 | ||||
| Con1 | Male | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/5 |
| Female | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/5 | |
| SSE2 | Male | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/5 |
| Female | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/5 | |
Table 4.
Clinical signs of male and female mice orally treated with 10 mL/kg of Samwhaing-sasimtang ethanol extract (1%)
| Material | Sex | Clinical signs | Hours after treatment | Days after treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 3 | 7 | 14 | |||
| Con1 | Male | Normal | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Female | Normal | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| SSE2 | Male | Normal | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Female | Normal | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
Table 5.
Body weights of male and female mice orally treated with 10 mL/kg of Samwhaing-sasimtang ethanol extract (1%)
| Material | Sex | Number of animals | Days after treatment | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | |||
| Con1 | Male | 5 | 20.72±0.65 | 22.98±0.75 | 27.05±1.08 | 29.21±1.35 | 33.54±1.88 |
| Female | 5 | 19.83±0.52 | 20.84±0.95 | 25.09±1.25 | 28.11±1.54 | 30.51±2.43 | |
| SSE2 | Male | 5 | 20.88±0.58 | 22.18±0.93 | 26.55±1.51 | 28.05±1.94 | 32.14±1.89 |
| Female | 5 | 20.21±0.59 | 20.89±0.92 | 24.92±1.35 | 27. 10±1.67 | 29.39±2.21 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download