Abstract
BACKGROUND/OBJECTIVE
Myocardial cell death due to occlusion of the coronary arteries leads to myocardial infarction, a subset of coronary heart disease (CHD). Dietary fiber is known to be associated with a reduced risk of CHD, the underlying mechanisms of which were suggested to delay the onset of occlusion by ameliorating risk factors. In this study, we tested a hypothesis that a beneficial role of dietary fiber could arise from protection of myocardial cells against ischemic injury, manifested after occlusion of the arteries.
MATERIALS/METHODS
Three days after rats were fed apple pectin (AP) (with 10, 40, 100, and 400 mg/kg/day), myocardial ischemic injury was induced by 30 min-ligation of the left anterior descending coronary artery, followed by 3 hr-reperfusion. The area at risk and infarct area were evaluated using Evans blue dye and 2,3,5-triphenyltetrazolium chloride (TTC) staining, respectively. DNA nicks reflecting the extent of myocardial apoptosis were assessed by TUNEL assay. Levels of cleaved caspase-3, Bcl-2, and Bax were assessed by immunohistochemistry.
RESULTS
Supplementation of AP (with 100 and 400 mg/kg/day) resulted in significantly attenuated infarct size (IS) (ratio of infarct area to area at risk) by 21.9 and 22.4%, respectively, in the AP-treated group, compared with that in the control group. This attenuation in IS showed correlation with improvement in biomarkers involved in the apoptotic cascades: reduction of apoptotic cells, inhibition of conversion of procaspase-3 to caspase-3, and increase of Bcl-2/Bax ratio, a determinant of cell fate.
CONCLUSIONS
The findings indicate that supplementation of AP results in amelioration of myocardial infarction by inhibition of apoptosis. Thus, the current study suggests that intake of dietary fiber reduces the risk of CHD, not only by blocking steps leading to occlusion, but also by protecting against ischemic injury caused by occlusion of the arteries.
Myocardial infarction, defined by pathology as myocardial cell death due to prolonged ischemia, occurs by a typical sequence of the following events: a high level of low-density lipoprotein (LDL) in the serum causes accumulation of LDL in the arterial intima, and oxidation of LDL in the intima results in formation of atherosclerotic plaques. Subsequent erosion and/or rupture of the plaques lead to formation of thrombus in the lesions, which leads to occlusion of the arteries, myocardial ischemia, and finally myocardial cell death by necrosis and apoptosis [1,2,3,4]. In prospective cohort studies, intake of dietary fiber, particularly water-soluble fiber from fruits and cereals, was shown to be associated with a reduced risk of coronary heart disease (CHD), including myocardial infarction [5,6,7,8]. Several mechanisms were proposed to explain how dietary fiber reduces the risk of CHD, in which dietary fiber exerts protective effects by blocking the steps leading to occlusion of the coronary arteries, such as decrease in LDL and C-reactive protein levels and attenuation in the blood pressure [9,10]. In our previous study, we demonstrated that supplementation of a dietary fiber, arabinoxylan, was effective in attenuating brain injury under ischemic conditions [11]. The findings suggest that intake of dietary fiber might also reduce myocardial injury under ischemic conditions by attenuating myocardial cell death after occlusion of the coronary arteries. To test this hypothesis, we performed experiments to determine whether apple pectin (AP) protected against myocardial cell injury in a rat model of ischemia/reperfusion. AP was selected as a model fiber because intake of apple showed an inverse association with the risk of CHD [12], and intake of apple [13] or AP [14,15] exerted a cholesterol-lowering effect. However, the question of whether or not intake of AP for a short period not long enough to affect atherosclerosis could attenuate myocardial injury induced by occlusion of the coronary arteries was not investigated.
Highly esterified (70-75%) apple pectin (AP) with a molecular weight of 30,000-100,000 (catalog number 76282) and other reagents were purchased from Sigma (St. Louis, MO, USA), unless stated otherwise. A fiber-free diet (950 g/kg control diet) consisting of casein (250 g/kg), corn starch (482.5 g/kg), sucrose (100 g/kg), soybean oil (70 g/kg), mineral mix (35 g/kg), vitamin mix (10 g/kg), and choline bitartrate (2.5 g/kg) was prepared as described previously [16], and provided by Unifaith Inc. (Seoul, Korea) (Table 1). Fifty grams of corn starch (Sigma, St. Louis, MO, USA) was added to 950 g of the fiber-free diet to make 1 kg of a basal diet. For preparation of 1 kg of an AP diet from the fiber-free diet, 0.2, 0.8, 2, and 8 g of apple pectin pre-mixed in the corn starch with the total amount of 50 g was incorporated in 950 g of the fiber-free diet to yield 1 kg of 10, 40, 100, and 400 AP diet, respectively.
Rats were randomly assigned to one of the three groups and acclimatized for three days with the basal diet: (1) sham (n = 5), (2) control (n = 5), and (3) AP-treated group (10, 40, 100, and 400 mg/kg/day) (n = 8, 6, 5, and 7, respectively). Eight rats were assigned to the control group and the AP-treated group at the beginning of the experiment. However, some rats expired during the occlusion period due to experimental severity. Thus, the number of rats in each group represents the number of rats that survived until the end of the experiments. In the AP-treated group, rats received the respective dose of AP incorporated in the AP diet for another three days before occlusion. For example, 48 g of corn starch and 2 g of AP were mixed to make 50 g of a mixture of corn starch and AP when rats weighing 300 g were fed 100 mg/kg/day AP and a rat consumed 15 g of AP diet per day. Once the rats had consumed all of the AP diet, a more basal diet was provided ad libitum. In the control group, rats received the basal diet only. In the sham group, experimental procedures were the same as in the control group, except that there was no ligation.
The neutral monosaccharide composition of AP was determined, as described previously [17]. Briefly, AP was hydrolyzed with 2M trifluoroacetic acid, reduced with 0.5 M sodium borohydride, and acetylated with acetic anhydride. The composition of generated alditol acetates was assessed by gas chromatography analysis.
Eight-week-old male Sprague Dawley (SD) rats were purchased from Samtaco Inc. (Osan, Korea). Experiments were performed according to the guidelines for the animal care and use of laboratory animal protocols approved by the Institutional Animal Care and Research Advisory Committee of Catholic University, Daegu, Korea (IRB number: 2012-0416-CU-AEC-10-Y). Animals were housed with a chow diet and water available ad libitum under diurnal lighting conditions and in a temperature-controlled environment until the start of the experiment.
Myocardial infarction was generated by ligating the left anterior descending coronary artery (LAD) in male SD rats (~300 g), as described previously [18,19]. Briefly, rats were anesthetized with intramuscular injections of ketamine (100 mg/kg) and xylazine (5 mg/kg). The rats were ventilated with air after intubation. The LAD was ligated at approximately 5 mm down from the aortic origin. In ischemia-reperfusion experiments, the hearts were ligated for 30 min and reperfused for 3 hr. During surgery, rectal temperature was maintained between 37 ± 0.5℃ with a thermostatically controlled warming plate (Harvard Apparatus, Holliston, MA).
Infarct size was assessed by 2,3,5-triphenyltetrazolium chloride (TTC) staining [18,19]. Briefly, the LAD was religated after 30 min-ischemia/3 hr-reperfusion. Evans blue dye was then infused, and area at risk (AAR) showing no infiltration of Evans blue dye was assessed. Four slices prepared by cutting the myocardium were stained in TTC, and infarct area (IA), the region not stained with TTC, was assessed. Areas of left ventricle (LV), AAR, and IA were determined by computerized planimetry using Image J software, from which infarct size (IS) and risk size (RS) were defined as a percentage of IA to AAR and of AAR to LV, respectively.
For measurement of DNA nicks, terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) staining was performed for detection of myocardial apoptosis [18,19]. Briefly, sections prepared on slides were treated with proteinase K and hydrogen peroxide, and then incubated with terminal deoxynucleotidyl transferase (TdT) and 5-bromo-2'-deoxyuridine-5'-triphosphate (Br-dUTP). For counter-staining, the sections were stained with methyl green. To determine the percentage of apoptotic cells, micrographs of TUNEL-positive nuclei and methyl green-stained nuclei were captured using an Olympus microscope and counted using ImageJ software (ImageJ version 1.43r; NIH) from 10 to 20 random fields at 400× magnification [20].
Immunohistochemical techniques were utilized for assessment of the presence of Bcl-2, Bax, and cleaved caspase-3 [18,19]. Briefly, endogenous peroxidases in the sections were blocked with H2O2, and the sections were then blocked with bovine serum albumin and normal goat serum. Rabbit polyclonal anti-Bcl-2, anti-Bax (1:50 dilution, Santa Cruz Biotechnology Inc., Santa Cruz, CA), or anti-cleaved caspase-3 primary antibody (1:50 dilution, Cell Signaling, Beverly, MA) was added, and the sections were stained using a Vectastain Elite ABC kit (Vector Laboratories, Burlington, ON), in which color was developed with 3,3'-diaminobenzidine tetrahydrochloride (DAB; Roche, Mannheim, Germany). The sections were evaluated at a 200× magnification. The intensity of DAB staining was measured using Image J software. Relative values for the intensity were used by setting the value for the sham group at 1.
Values were expressed as mean ± SEM. Statistical analysis for a single comparison was performed using Student's t-test. When necessary, multiple comparisons were performed using one-way ANOVA, followed by the Tukey post hoc test using SPSS software (IBM SPSS Statistics; version 19, Armonk, NY, USA). P < 0.05 was considered as indicating statistical significance.
Composition of the neutral monosaccharides constituting AP was assessed; AP was hydrolyzed to its constituent monosaccharides with TFA, reduced to the corresponding alditols with sodium borohydride, and acetylated to give the corresponding alditol acetates. Results of identification and quantification of the resultant alditol acetates by gas chromatography, with allose being used as an internal standard [17], are as follows: 1.8% rhamnose (rha), 1.5% arabinose (ara), 1.3% xylose (xyl), 6.5% galactose (gal), and 3.7% glucose (glc). Fraeye et al. [21], who also analyzed AP, purchased from Fluka, using TFA hydrolysis followed by separation and detection with HPAEC-PAD (High Performance Anion Exchange Chromatography with Pulsed Amperometric Detection), reported the composition to be 1.2% rha, 2.1% ara, 0.9% xyl, 4.6% gal, and 3.0% glc, which is similar to that obtained in the current study.
To determine a range of effective doses in protecting the heart from ischemia/reperfusion injury, the effect of AP intake on the reduction of infarct size was examined at four different doses (with 10, 40, 100 and 400 mg/kg/day) in a rat model (Fig. 1). Fed with AP for three days, the rats underwent 30 minischemia and a subsequent 3 hr-reperfusion. Evans blue dye was then infused in order to delineate the area at risk (AAR), whereby blood did not circulate during ischemia. Subsequently, the hearts were cut into four slices, and the slices were stained with TTC in order to delineate the infarct area (IA), where cardiomyocytes died [18,19] (Fig. 1A, B). To quantify the results, risk size, defined as a ratio of AAR to the left ventricle (LV), [RS(AAR/LV)], was assessed first. RS(AAR/LV) in the AP-treated groups (with 10, 40, 100, and 400 mg/kg/day) did not differ significantly from that in the control group (P > 0.05), showing that the surgical procedures for ligation of LAD were performed consistently (Fig. 1C). These results assure that infarct size, defined as a ratio of IA to AAR [IS(IA/AAR)], assessed for each group, was also reliable due to experimental consistency. Intake of AP 100 and 400 mg/kg/day resulted in significantly reduced IS(IA/AAR), compared with that in the control group (42.5 ± 3.0 and 42.2 ± 2.4%, respectively, versus 55.4 ± 1.57%, P < 0.05), whereas intake of AP 10 and 40 mg/kg/day did not significantly affect IS(IA/AAR) (P > 0.05) (Fig. 1C).
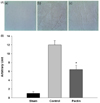
TUNEL staining was used to determine whether attenuation of IS(IA/AAR) arose from an antiapoptotic effect of AP, as TUNEL staining has previously been applied for detection of apoptotic cells in mouse models of myocardial infarction [22,23,24]. Apoptotic cells in AAR were assessed in two separate regions, infarct area (IA) and border zone (BZ), defined as the region where IA is excluded from AAR because the pattern of apoptosis in the two regions could be different [25]. Representative sections in the AP-treated group in IA and BZ showed that apoptotic cells in both regions tended to decrease when the rats were treated with AP 100 mg/kg/day, compared with those in the control group (Fig. 2A). To quantify the results, the ratio of apoptotic cells to total cells was assessed (Fig. 2B). Only in BZ, the ratio was significantly reduced in the AP-treated group 100 mg/kg/day, compared with that in the control group (11.2 ± 1.9 versus 23.8 ± 4.5, P < 0.05).
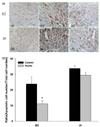
To support the results of TUNEL staining, the amount of cleaved caspase-3 was assessed by immunohistochemistry [26,27] (Fig. 3). In the apoptotic pathways, cleaved (active) caspase-3, derived from procaspase-3 by removal of prodomain, cleaves a restricted set of target proteins, resulting in cell death by apoptosis [28]. Thus, increase in cleaved caspase-3 induces apoptotic cell death [29,30]. A representative section for the AP-treated group in BZ showed that the amount of cleaved caspase-3 reflected by the color intensity tended to be reduced in the AP-treated group, compared with that in the control group (Fig. 3A). To quantify the results, the amount of cleaved caspase-3 in the control and AP-treated groups was assessed by setting that in the sham group at 1 (Fig. 3B). The amount of cleaved caspase-3 was significantly reduced in the AP-treated group (with 100 mg/kg/day), compared with that in the control group (6.4 ± 0.9% versus 11.9 ± 0.9%, P < 0.05).
Expression levels of Bcl-2 and Bax, located upstream of caspase-3 in the apoptotic pathways, were evaluated by immunohistochemistry. Bcl-2 and Bax are the key regulatory components of the cell death process, in which Bcl-2 and Bax are antiapoptotic and proapoptotic, respectively [31,32,33] (Fig. 4). In representative sections of the AP-treated group, the expression level of Bcl-2 tended to increase in the AP-treated group (with 100 mg/kg/day), compared with that in the control group (Fig. 4A). In contrast, the expression level of Bax tended to decrease in the AP-treated group (with 100 mg/kg/day), compared with that in the control group. This was also confirmed with quantitative assessment, for which Bcl-2/Bax ratio was used because this ratio determines whether the cells live or die [34] (Fig. 4B). The ratio was significantly increased in the AP-treated group (with 100 mg/kg/day), compared with that in the control group (1.03 ± 0.03 versus 0.51 ± 0.04%, P < 0.05).
Myocardial cell death in myocardial infarction accompanies occlusion of the coronary arteries. Although it usually takes several decades for the occlusion to occur by disruption of the atherosclerotic plaques, a cascade of events leading to myocardial cell death takes place in a relatively short term once the arteries are blocked [3,35]. Occlusion of the coronary arteries supplying blood to the heart causes deficiency of oxygen and glucose in cardiomyocytes, leading to ischemic condition. Consequent blockade of ATP production by oxidative phosphorylation is responsible for the cell death [36], under which condition reperfusion of the occluded arteries is the most prevalent treatment for prevention of extensive cell death [37]. Augmenting the reperfusion therapy, attenuation of apoptosis is another option for reduction of cell death, because apoptosis also contributes to cell death, in addition to necrosis [35,38]. In the apoptotic pathways, ischemia causes translocation of Bax into the outer mitochondrial membrane, resulting in release of cytochrome c from the mitochondria, which is counteracted by Bcl-2. This event causes activation of procaspase-3 into active caspase-3 (cleaved caspase-3), leading to apoptotic cell death [33,35,39]. In the context of the apoptotic pathways, intake of AP in the rat model of myocardial ischemia/reperfusion resulted in increased Bcl-2/Bax ratio, subsequent activation of procaspase-3 to active (cleaved) caspase-3, and reduction of DNA cleavage, resulting in attenuation of apoptosis. Finally, reduction of apoptosis correlates with protection of the heart against injury from ischemia/reperfusion.
Once ingested, pectin is cleaved into constituent monosaccharides, including arabinose and xylose, in the large intestine [40]. They are subsequently fermented by microflora, yielding short chain fatty acids (SCFA), such as butyrate, propionate, and acetate [40,41]. The efficacy of pectin in exerting protection against ischemic injury might be mediated by SCFA generated because administration of butyrate by intraperitoneal injection prior to occlusion protected against myocardial injury in a rat model of ischemia/reperfusion [18]. However, a dose of AP exerting protection against ischemic injury in this study (2 g/kg diet) is too small compared with a dose of AP used to elevate butyrate content two fold (50 g/kg diet) [41]. Thus, protection by pectin ingestion might be mediated by constituent monosaccharides like arabinose as well as by its metabolites like butyrate. This hypothesis would be tested in the future.
In this study, we demonstrated that intake of pectin protected the heart against ischemic insult, and these findings have implications in elucidating a role of dietary fiber in reducing the risk of CHD. Traditionally, it has been thought that intake of dietary fiber contributed to reduction of the risk of CHD by delaying occlusion of the coronary arteries, not by improving myocardial cell survival after the onset of occlusion [8,9]. This conclusion can also be applied to pectin; intake of pectin lowered LDL cholesterol in the serum [42], protected against progression of atherosclerosis [43], and rendered fibrin networks more permeable [44], all of which are related to delaying the onset of occlusion. However, beneficial effects of AP identified from this study cannot be explained by traditional roles of AP in reducing the risk of CHD because duration of administration of AP (three days) is too short for development of a spontaneous occlusion of LAD. Thus, the findings of the current study suggest a more direct role of dietary fiber, in addition to indirect roles already accepted, in order to explain the beneficial effects of dietary fiber in prevention of CHD, including myocardial infarction.
Figures and Tables
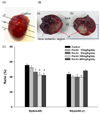 | Fig. 1
Effect of apple pectin (AP) on infarct size in rats. Rats underwent 30 min-ischemia/3 hr-reperfusion, followed by injection of Evans blue through the jugular vein, from which area at risk (AAR) and non-ischemic region were defined as the area with Evans blue not infiltrated and the area with Evans blue infiltrated, respectively. (A) The myocardium was then excised into four pieces, approximately 3 mm thick, and two pieces (a-b and b-c) were used for quantification of infarct area. (B) The slices were stained with TTC, from which area at risk (AAR), infarct area (IA), border zone (BZ), and non-ischemic (Non-ischemic) heart tissue were defined: (a) Vehicle-treated control group; (b) Extract-treated group. C. Infarct size [IS(IA/AAR)] and risk size [(RS(AAR/LV)) were expressed as a percentage of IA to AAR and of AAR to the left ventricle (LV), respectively. AP (with 10, 40, 100, and 400 mg/kg/day) was fed for three days prior to occlusion. The numbers of rats used in the control and AP (with 10, 40, 100, and 400 mg/kg/day)-treated groups were 5, 8, 6, 5, and 7, respectively. *P < 0.05 vs. control group. |
 | Fig. 2
Effect of apple pectin (AP) on apoptosis. (A) Photomicrographs of myocardial tissue sections showing TUNEL staining (400×): (a), (d) sham group; (b), (e) control group; (c), (f) AP-treated group (with 100 mg/kg/day); (a)~(c) and (d)~(f) were taken from the border zone (BZ) and infarct area (IA), respectively. (B) Quantitative analysis of TUNEL-positive cells. The ratio of apoptotic cells to the total cells is presented. The numbers of rats used in the sham, control, and AP-treated groups were 5, 5, and 5, respectively. *P < 0.05 vs. control group. |
 | Fig. 3
Effect of apple pectin (AP) on activation of procaspase-3 to cleaved caspase-3. (A) Photomicrographs of myocardial tissue sections showing cleaved caspase-3 expression (200×): (a), sham group; (b), control group; (c), AP-treated group (with 100 mg/kg/day). Photomicrographs were randomly taken from area at risk (AAR). (B) Quantitative analysis of cleaved caspase-3 protein. Arbitrary units were used. The numbers of rats used in the sham, control, and AP-treated groups were 5, 5 and 5, respectively. *P < 0.05 vs. control group. |
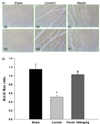 | Fig. 4
Effect of apple pectin (AP) on expression of Bcl-2 and Bax. (A) Photomicrographs of myocardial tissue sections showing Bcl-2 and Bax expression (200×): (a), (d) sham group; (b), (e) control group; (c), (f) AP-treated group (with 100 mg/kg/day). Photomicrographs were randomly taken from BZ. (a)~(c) and (d)~(f) represent Bcl-2 and Bax, respectively. (B) Quantitative analysis of Bcl-2 and Bax. Bcl-2/Bax ratio was used. The numbers of rats used in the sham, control, and AP-treated groups were 5, 5, and 5, respectively. *P < 0.05 control vs. sham group, #P < 0.05 AP-treated vs. control group. |
Table 1
Composition of Experimental Diets

aFiber-free diet was purchased in a pre-mixed form.
bOne kg of basal diet was prepared by addition of 50 g of corn starch to 950 g of fiber-free diet.
c10, 40, 100, and 400 AP diets refer to the corresponding dose of AP given per kg of rat per day.
dAP was calculated, based on data indicating that a 300 g rat consumed 15 g of pectin diet/day.
References
1. Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007; 116:2634–2653.

2. Badimon L, Padró T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care. 2012; 1:60–74.

3. Chapman MJ. From pathophysiology to targeted therapy for atherothrombosis: a role for the combination of statin and aspirin in secondary prevention. Pharmacol Ther. 2007; 113:184–196.

4. Brener SJ. Insights into the pathophysiology of ST-elevation myocardial infarction. Am Heart J. 2006; 151:S4–S10.

5. Eshak ES, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Wakai K, Tamakoshi A. JACC Study Group. Dietary fiber intake is associated with reduced risk of mortality from cardiovascular disease among Japanese men and women. J Nutr. 2010; 140:1445–1453.

6. Pereira MA, O'Reilly E, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med. 2004; 164:370–376.
7. Bazzano LA, He J, Ogden LG, Loria CM, Whelton PK. National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Dietary fiber intake and reduced risk of coronary heart disease in US men and women: the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Intern Med. 2003; 163:1897–1904.

8. Shin D. Analysis of dietary insoluble and soluble fiber contents in school meal. Nutr Res Pract. 2012; 6:28–34.

9. Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2008; 108:1716–1731.

11. Han HS, Jang JH, Jang JH, Choi JS, Kim YJ, Lee C, Lim SH, Lee HK, Lee J. Water extract of Triticum aestivum L. and its components demonstrate protective effect in a model of vascular dementia. J Med Food. 2010; 13:572–578.

12. Hansen L, Dragsted LO, Olsen A, Christensen J, Tjønneland A, Schmidt EB, Overvad K. Fruit and vegetable intake and risk of acute coronary syndrome. Br J Nutr. 2010; 104:248–255.

13. Jensen EN, Buch-Andersen T, Ravn-Haren G, Dragsted LO. Mini-review: The effects of apples on plasma cholesterol levels and cardiovascular risk - a review of the evidence. J Hortic Sci Biotechnol. 2009; 84:34–41.

14. Brouns F, Theuwissen E, Adam A, Bell M, Berger A, Mensink RP. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur J Clin Nutr. 2012; 66:591–599.

15. Sánchez D, Muguerza B, Moulay L, Hernández R, Miguel M, Aleixandre A. Highly methoxylated pectin improves insulin resistance and other cardiometabolic risk factors in Zucker fatty rats. J Agric Food Chem. 2008; 56:3574–3581.

16. Nishimura N, Tanabe H, Sasaki Y, Makita Y, Ohata M, Yokoyama S, Asano M, Yamamoto T, Kiriyama S. Pectin and high-amylose maize starch increase caecal hydrogen production and relieve hepatic ischaemia-reperfusion injury in rats. Br J Nutr. 2012; 107:485–492.

17. Melton LD, Smith BG. Determination of neutral sugars by gas chromatography of their alditol acetates. Curr Protoc Food Analyt Chem. 2001; E3.2.1–E3.2.13.

18. Lim SH, Song KS, Lee JW. Butyrate and propionate, short chain fatty acids, attenuate myocardial damages by inhibition of apoptosis in a rat model of ischemia-reperfusion. J Korean Soc Appl Biol Chem. 2010; 53:570–577.

19. Lim SH, Lee J. Methanol extract of Cassia mimosoides var. nomame attenuates myocardial injury by inhibition of apoptosis in a rat model of ischemia-reperfusion. Prev Nutr Food Sci. 2012; 17:177–183.

20. Ren J, Babcock SA, Li Q, Huff AF, Li SY, Doser TA. Aldehyde dehydrogenase-2 transgene ameliorates chronic alcohol ingestion-induced apoptosis in cerebral cortex. Toxicol Lett. 2009; 187:149–156.

21. Fraeye I, Duvetter T, Verlent I, Sila DN, Hendrickx M, Van Loey A. Comparison of enzymatic de-esterification of strawberry and apple pectin at elevated pressure by fungal pectinmethylesterase. Innov Food Sci Emerg Technol. 2007; 8:93–101.

22. Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, Feelisch M, Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation [corrected]. Proc Natl Acad Sci U S A. 2004; 101:11471–11476.

23. Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005; 11:305–311.

24. Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005; 11:1096–1103.

25. Krijnen PA, Nijmeijer R, Meijer CJ, Visser CA, Hack CE, Niessen HW. Apoptosis in myocardial ischaemia and infarction. J Clin Pathol. 2002; 55:801–811.

26. Zidar N, Dolenc-Strazar Z, Jeruc J, Stajer D. Immunohistochemical expression of activated caspase-3 in human myocardial infarction. Virchows Arch. 2006; 448:75–79.

27. Rabkin SW. Apoptosis in human acute myocardial infarction: the rationale for clinical trials of apoptosis inhibition in acute myocardial infarction. Sch Res Exch. 2009; 979318.

29. Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, Green DR. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005; 310:66–67.

30. Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006; 311:847–851.

31. Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001; 26:61–66.

32. van Empel VP, Bertrand AT, Hofstra L, Crijns HJ, Doevendans PA, De Windt LJ. Myocyte apoptosis in heart failure. Cardiovasc Res. 2005; 67:21–29.

34. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993; 74:609–619.

35. Abbate A, Bussani R, Amin MS, Vetrovec GW, Baldi A. Acute myocardial infarction and heart failure: role of apoptosis. Int J Biochem Cell Biol. 2006; 38:1834–1840.

36. Zucchi R, Ghelardoni S, Evangelista S. Biochemical basis of ischemic heart injury and of cardioprotective interventions. Curr Med Chem. 2007; 14:1619–1637.

37. Cohen M, Boiangiu C, Abidi M. Therapy for ST-segment elevation myocardial infarction patients who present late or are ineligible for reperfusion therapy. J Am Coll Cardiol. 2010; 55:1895–1906.

38. Ahmad R, Javed S, Bhandari U. Antiapoptotic potential of herbal drugs in cardiovascular disorders: an overview. Pharm Biol. 2010; 48:358–374.

39. Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004; 95:957–970.

40. Fischbach MA, Sonnenburg JL. Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe. 2011; 10:336–347.

41. Aprikian O, Duclos V, Guyot S, Besson C, Manach C, Bernalier A, Morand C, Rémésy C, Demigné C. Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. J Nutr. 2003; 133:1860–1865.

42. Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999; 69:30–42.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download