INTRODUCTION
MATERIALS AND METHODS
ADA extract and mice
Behavioral tests
Measurement of acetylcholine (ACh), malondialdehyde (MDA), Aβ, and interleukin (IL)-1β
Statistics
RESULTS
The effects of ADA extract on various Aβ-induced changes after i.c.v. injection of Aβ1-42 into mice
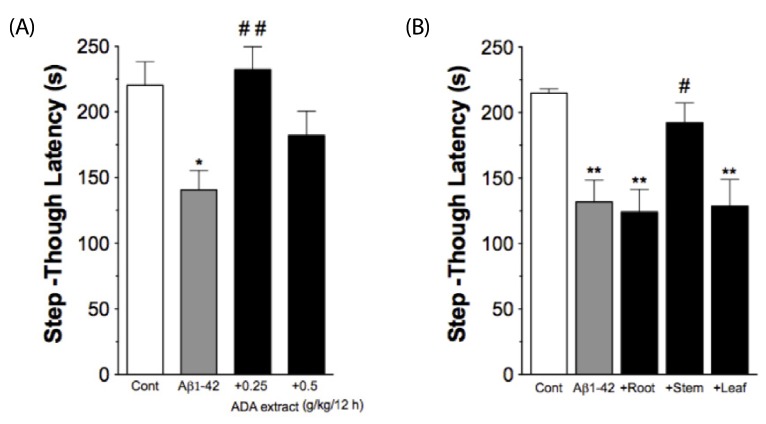 | Fig. 2
Protective effect of ADA stem extract on Aβ1-42-induced impairment of passive avoidance performance. (A). ADA stem extract (0.25 g/kg or 0.5 g/kg) was administered intra-gastrically every 12 hrs, five times (two times prior to Aβ1-42 treatment, and three times post-Aβ1-42 treatment). Passive avoidance test was performed one day after Aβ1-42 treatment. (B). Effects of different parts of ADA extract (0.25 g/kg) given with the same protocol on the Aβ1-42-induced impairment of passive avoidance performance. *P < 0.05, **P < 0.01 vs control. #P < 0.05, ##P < 0.01 vs Aβ1-42 alone. Data are expressed as mean ± SEM (n = 10-12). |
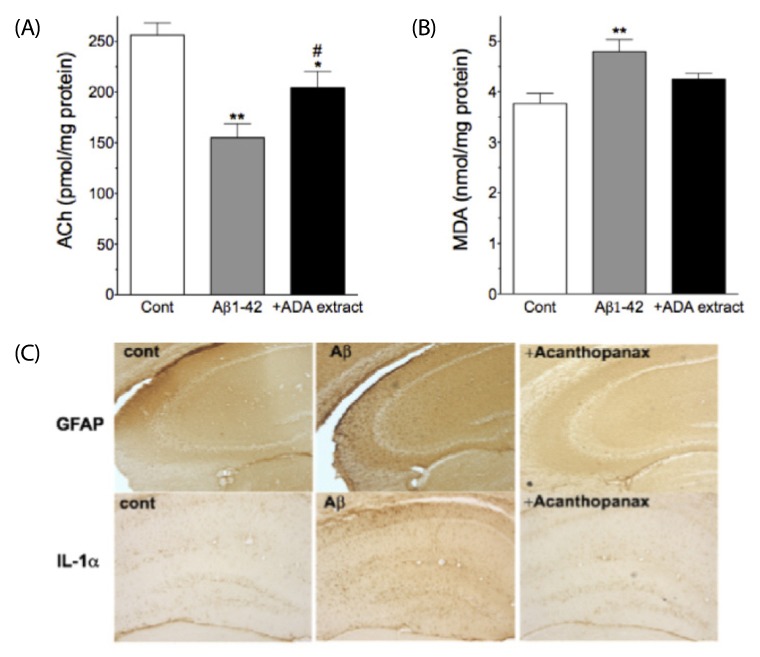 | Fig. 3
Protective effects of ADA stem extract on Aβ1-42-induced changes in cortical acetylcholine (ACh) and malondialdehyde (MDA) as well as hippocampal GFAP- and IL-1α-immunoreactivities. ADA stem extract (0.25 g/kg) was administered intra-gastrically every 12 hrs for six days (from one day before Aβ1-42 treatment to five days post-Aβ1-42 treatment), and evaluation was performed five days after Aβ1-42 treatment. *P < 0.05, **P < 0.01 vs control. #P < 0.05 vs Aβ 1-42 alone. Data are expressed as mean ± SEM (n = 10). |
The effects of chronic oral administration of ADA stem extract on APP/PS1 transgenic mice
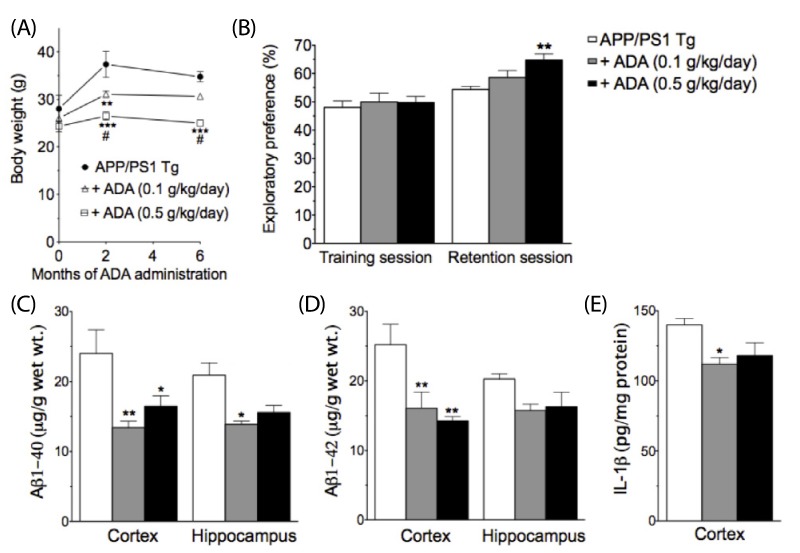 | Fig. 4
Effect of ADA stem extract treatment for six months on body weight (A), novel-object recognition task (B), brain Aβ (C,D) and IL-1β (E) levels in APP/PS1-transgenic mice at 12 months of age.
*P < 0.05, **P < 0.01, ***P < 0.001 vs control. #P < 0.05 vs ADA (0.1 g/kg/day). Data are expressed as mean ± SEM (a: 0 and two months, n = 4, 5, 5 for control, 0.1 g/kg/day, 0.5 g/kg/day, respectively; six months, n = 3, 5, 4 for control, 0.1 g/kg/day, 0.5 g/kg/day, respectively; b-e: n = 3, 5, 4 for control, 0.1 g/kg/day, 0.5 g/kg/day, respectively). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download