INTRODUCTION
MATERIAL AND METHODS
Chemicals and bioreagents
Collection of seaweed samples and preparation of their extract
Cell lines, cell culture and animals
In-vitro assay of proliferation
Matrigel tube formation assay
Migration of HUVECs in the wound-healing assay
Chorioallantoic membrane assay (CAM)
Corneal Neovascularization (CNV)
Record of body weight, ascites volume, cell number and peritoneal angiogenesis of EAT hearing mice treated with S. marginatum methanolic extract
Immunohistological analysis (H & E staining)
CD 31 Immunostaining
Statistical analysis
RESULTS
S. marginatum extract inhibits in vitro proliferation of EAT/ BeWo cells
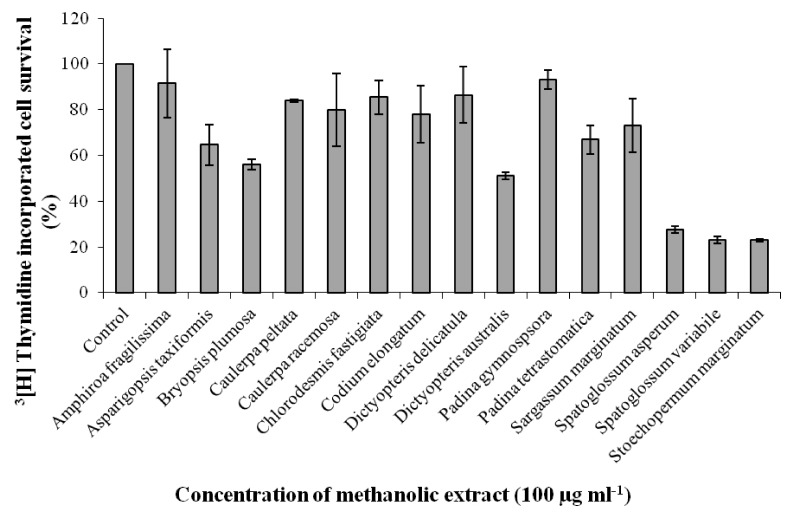 | Fig. 1
Effect of 15 seaweed methanolic extracts on proliferation of EAT cells in-vitro. EAT cells were plated in 12 well plates and incubated for 48 h. Seaweed extracts of 100 µg ml-1 concentration were added to the wells in triplicate prior to addition of 3[H] thymidine followed by incubation for another 48 h. The cells were trypsinized after two days and processed for scintillation counting. Values are presented as mean ± SD (n = 3). |
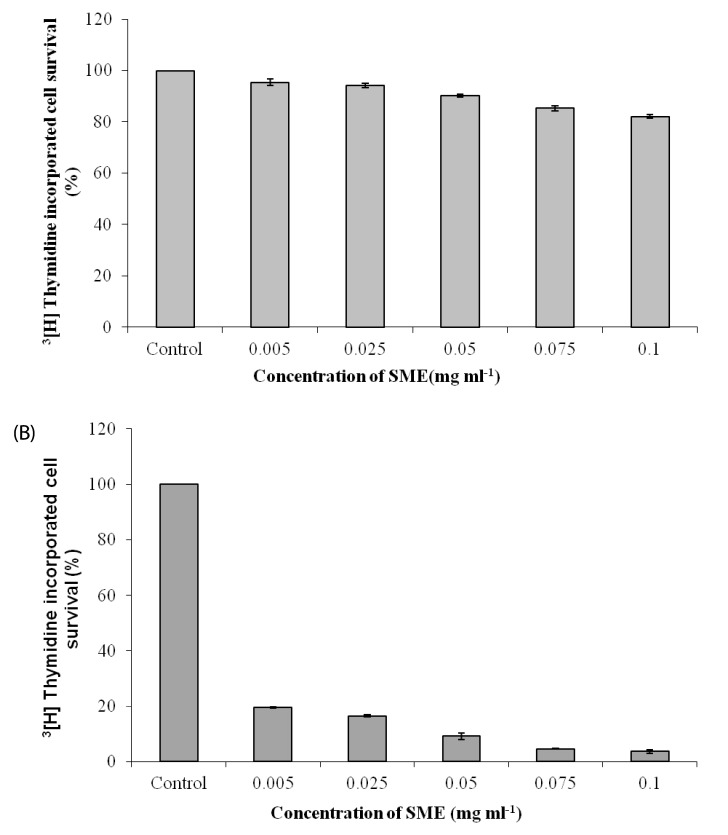 | Fig. 2
Effect of S. marginatum extract (SME) on proliferation of HEK-293 and BeWo cells in-vitro. (A) HEK-293 and (B) BeWo cells were plated in 12 well plates and incubated for 48h. SME in concentrations of 0.005, 0.025, 0.05, 0.075 and 0.1 mg ml-1 was added to the wells in triplicate prior to addition of 3[H] thymidine followed by incubation for another 48 h. The cells were trypsinized after two days and processed for scintillation counting. Values are presented as mean ± SD (n = 3). |
SME inhibits VEGF induced in-vitro tube formation of HUVEC's
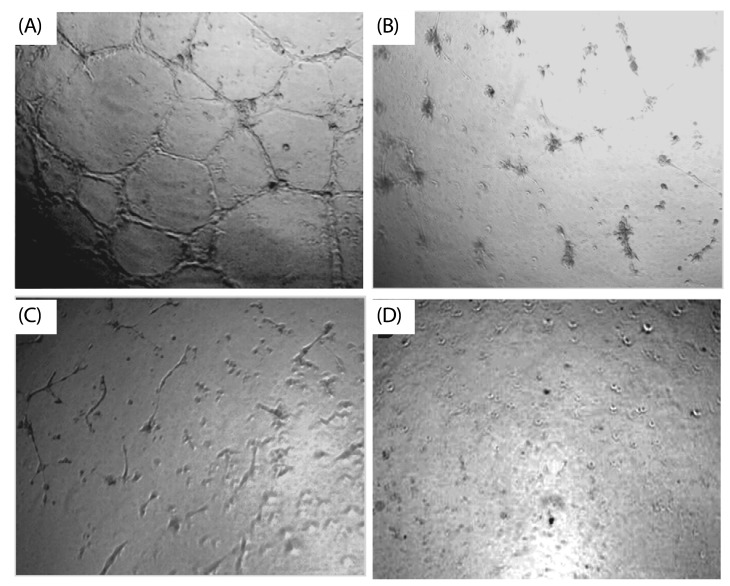 | Fig. 3
Inhibitory effect of S. marginatum extract (SME) on VEGF induced tube formation in-vitro. HUVEC's were seeded into the matrigel layer in a 96-well plate. (A) VEGF alone (+ ve control), (B) without VEGF (-ve control), (C) VEGF + SME (5 µg/ well), (D) VEGF + SME (10 µg/ well). The experiment was repeated three times with similar results (values are presented as mean ± S.D; n = 3). Three replicate fields of triplicate wells were digitally photographed. |
Effects of SME on HUVEC migration in the wound healing assay
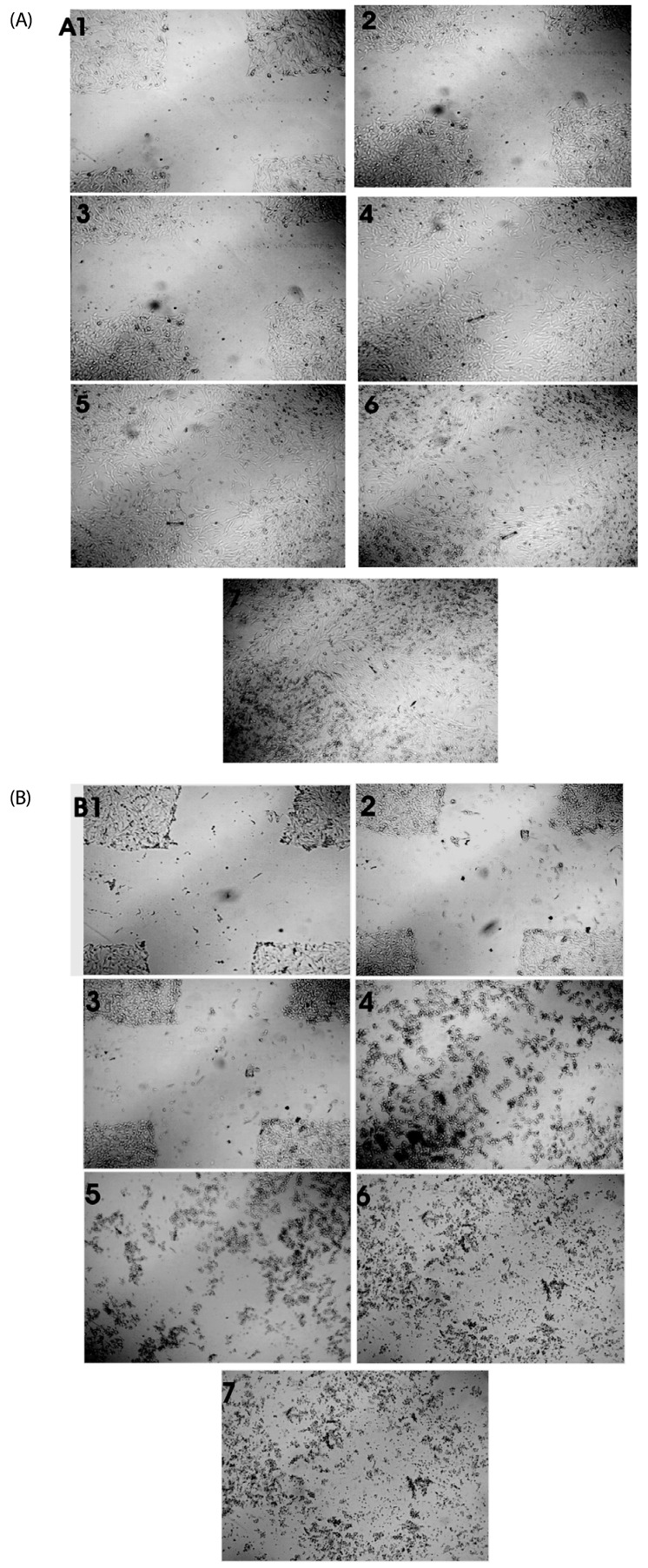 | Fig. 4
Effect of S. marginatum extract (SME) on HUVEC cell migration in an in-vitro scratch wound healing assay. The representative phase-contrast images show migration of cells into the wounded area. (A) Wound closure in control wells at 1) 0h, 2) 3h, 3) 6h, 4) 18h, 5) 24h, 6) 36h and 7) 42h. (B) Wound closure in SME (100 µg/ well) treated wells at 1) 0h, 2) 3h, 3) 6h, 4) 18h, 5) 24h, 6) 36h and 7) 42h. |
Angio-suppressive effect of SME on CAM and CNV
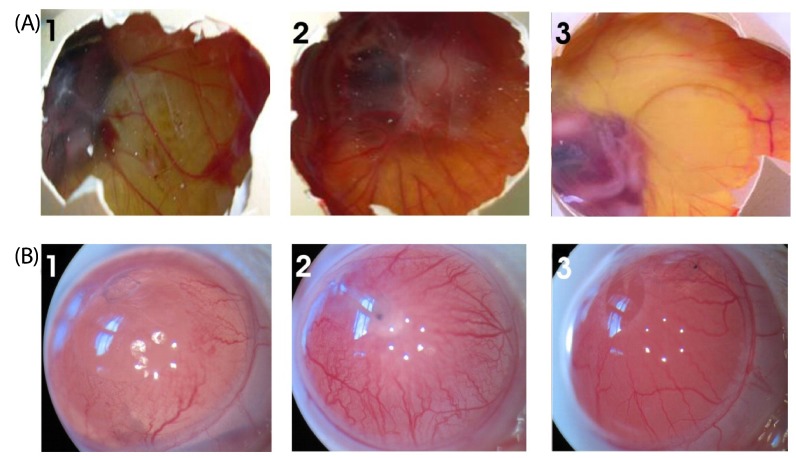 | Fig. 5
Effect of SME on blood vessel regression in the chick CAM and rat cornea assays in-vivo. (A) Photographs of VEGF-induced neovascularization observed in CAM: (1) Saline (- control), (2) VEGF alone (+ control), (3) VEGF + SME (100 µg) was applied to the CAM of 11-day-old chicken embryos. After incubation for 48h, the treated area was inspected for changes in neovascularization. The arrows indicate the treated area. The data shown represent the result of an experiment performed using a maximum of six eggs in each group. All photographs were taken at 40 × magnification. (B) Photographs of VEGF-induced neovascularization observed in rat corneas: (1) hydron polymer + VEGF (1 µg) (+ control), (2) hydron polymer alone (- control), and (3) hydron polymer + VEGF + SME (100 µg). After incubation for seven days, the corneas were photographed at 40 × magnification. |
In-vitro treatment with SME extract inhibits growth of EAT cells in the peritoneal angiogenesis assay
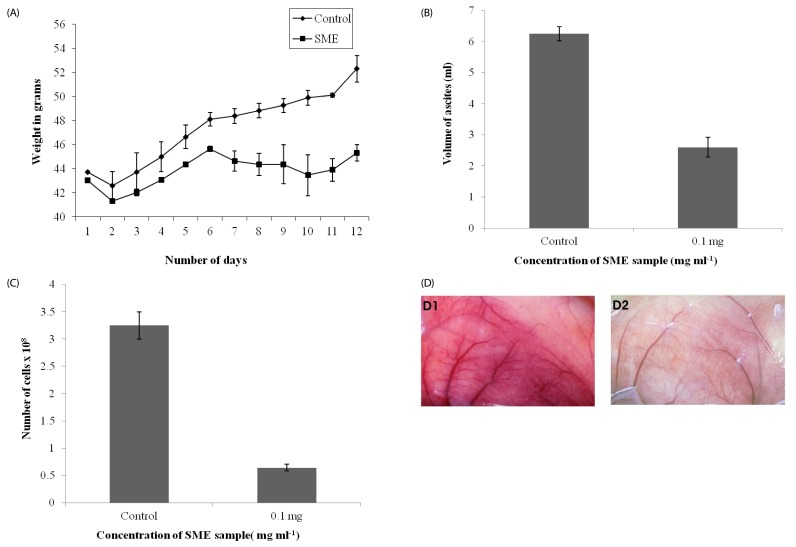 | Fig. 6
In-vivo inhibition of tumor growth and angiogenesis by S. marginatum extract (SME). (A) Body weights of EAT-bearing untreated mice or mice treated with SME were recorded. From the sixth day onward, SME (4 mg kg-1 body weight) was administered (i.p) every day for six days; the animals were sacrificed on the 12th day. (B) EAT cells were collected along with ascites fluid and measured, (C) Cells were counted using a haemocytometer, (D) The peritoneum of the animal was photographed: (1) Control, (2) SME treated. At least six mice were used in each group and the results obtained are an average of three individual experiments and mean of ± SD (n = 6 per group). |
SME inhibits formation of MVD and proliferation of endothelial cells
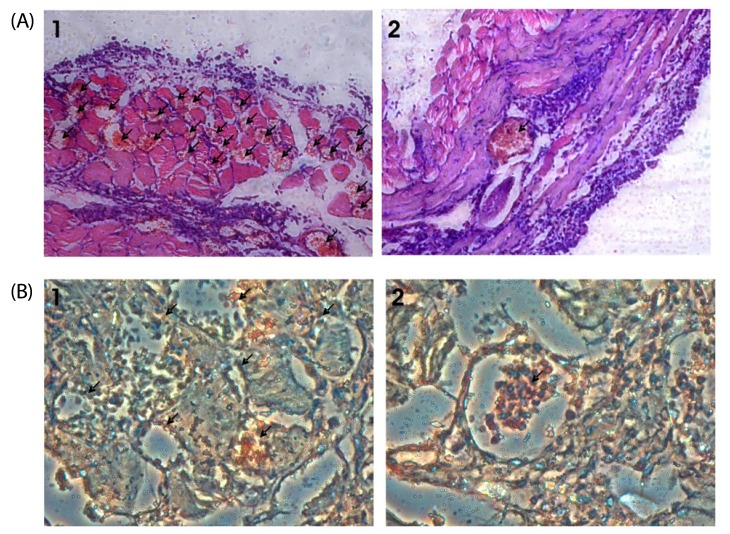 | Fig. 7
S. marginatum extract (SME) inhibits MVD and proliferation of endothelial cells in mouse peritoneum. (A) The peritoneums of control (1) as well as SME-treated (2) EAT-bearing mice were embedded in paraffin and 5 µm sections were made using a microtome. The sections were stained with hematoxylin and eosin and observed for microvessel density (40×). Arrows indicate the microvessels. (B) Paraffin sections (5 µm) of peritoneum of control (1) and SME (2) mice were immunostained with anti-CD31 (PECAM) anti-bodies. Arrows indicate the stained activated endothelial cells. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download