1. Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004; 164:1422–1426.

2. Avogaro A, Fadini GP, Gallo A, Pagnin E, de Kreutzenberg S. Endothelial dysfunction in type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2006; 16:Suppl 1. S39–S45.

3. Cubbon RM, Kahn MB, Wheatcroft SB. Effects of insulin resistance on endothelial progenitor cells and vascular repair. Clin Sci (Lond). 2009; 117:173–190.

4. Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003; 107:461–468.

5. Fadini GP, Avogaro A. Potential manipulation of endothelial progenitor cells in diabetes and its complications. Diabetes Obes Metab. 2010; 12:570–583.

6. Reinhard H, Jacobsen PK, Lajer M, Pedersen N, Billestrup N, Mandrup-Poulsen T, Parving HH, Rossing P. Multifactorial treatment increases endothelial progenitor cells in patients with type 2 diabetes. Diabetologia. 2010; 53:2129–2133.

7. Liao YF, Chen LL, Zeng TS, Li YM, Fan Y, Hu LJ, Ling Y. Number of circulating endothelial progenitor cells as a marker of vascular endothelial function for type 2 diabetes. Vasc Med. 2010; 15:279–285.

8. Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest. 2000; 106:571–578.

9. Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005; 115:572–583.

10. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003; 348:593–600.

11. Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005; 45:1449–1457.

12. Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004; 53:195–199.
13. Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation. 2003; 108:1527–1532.
14. Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR, Huang PH, Liu PL, Chen YL, Chen JW. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007; 56:1559–1568.

15. Sheu ML, Ho FM, Yang RS, Chao KF, Lin WW, Lin-Shiau SY, Liu SH. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol. 2005; 25:539–545.

16. Yoshizumi M, Abe J, Tsuchiya K, Berk BC, Tamaki T. Stress and vascular responses: atheroprotective effect of laminar fluid shear stress in endothelial cells: possible role of mitogen-activated protein kinases. J Pharmacol Sci. 2003; 91:172–176.

17. Uruno A, Sugawara A, Kudo M, Satoh F, Saito A, Ito S. Stimulatory effects of low-dose 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor fluvastatin on hepatocyte growth factor-induced angiogenesis: involvement of p38 mitogen-activated protein kinase. Hypertens Res. 2008; 31:2085–2096.

18. McGinn S, Saad S, Poronnik P, Pollock CA. High glucose-mediated effects on endothelial cell proliferation occur via p38 MAP kinase. Am J Physiol Endocrinol Metab. 2003; 285:E708–E717.

19. Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989; 274:532–538.

20. Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am J Clin Nutr. 2004; 79:47–53.

21. Martin KR, Wu D, Meydani M. The effect of carotenoids on the expression of cell surface adhesion molecules and binding of monocytes to human aortic endothelial cells. Atherosclerosis. 2000; 150:265–274.

22. Kim GY, Kim JH, Ahn SC, Lee HJ, Moon DO, Lee CM, Park YM. Lycopene suppresses the lipopolysaccharide-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and nuclear factor-kappaB. Immunology. 2004; 113:203–211.

23. Chan CM, Fang JY, Lin HH, Yang CY, Hung CF. Lycopene inhibits PDGF-BB-induced retinal pigment epithelial cell migration by suppression of PI3K/Akt and MAPK pathways. Biochem Biophys Res Commun. 2009; 388:172–176.

24. Van Craenenbroeck EM, Conraads VM. Endothelial progenitor cells in vascular health: focus on lifestyle. Microvasc Res. 2010; 79:184–192.

25. Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004; 24:1442–1447.

26. Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001; 88:167–174.

27. Lin CY, Huang CS, Hu ML. The use of fetal bovine serum as delivery vehicle to improve the uptake and stability of lycopene in cell culture studies. Br J Nutr. 2007; 98:226–232.

28. Azuma K, Kawamori R, Toyofuku Y, Kitahara Y, Sato F, Shimizu T, Miura K, Mine T, Tanaka Y, Mitsumata M, Watada H. Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arterioscler Thromb Vasc Biol. 2006; 26:2275–2280.

29. Wang H, Wang L, Li KZ, Ling R, Sun BH, Ma FC, Li XJ. Culture and identification of endothelial progenitor cells from human peripheral blood. Chin J Clin Rehabil. 2006; 10:47–49.
30. Wang HX, Zhao S, Li BG, Mao H, Shao SY. Effects of high-glucose on proliferation and apoptosis in endothelial progenitor cells of type 2 diabetes mellitus. Chin J Pathophysiol. 2007; 23:2210–2213.
31. Kuwana M, Okazaki Y, Kodama H, Satoh T, Kawakami Y, Ikeda Y. Endothelial differentiation potential of human monocyte-derived multipotential cells. Stem Cells. 2006; 24:2733–2743.

32. Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002; 106:2781–2786.

33. Nakamura N, Naruse K, Kobayashi Y, Matsuki T, Hamada Y, Nakashima E, Kamiya H, Hata M, Nishikawa T, Enomoto A, Takahashi M, Murohara T, Matsubara T, Oiso Y, Nakamura J. High glucose impairs the proliferation and increases the apoptosis of endothelial progenitor cells by suppression of Akt. J Diabetes Investig. 2011; 2:262–270.

34. Gianetti J, Pedrinelli R, Petrucci R, Lazzerini G, De Caterina M, Bellomo G, De Caterina R. Inverse association between carotid intima-media thickness and the antioxidant lycopene in atherosclerosis. Am Heart J. 2002; 143:467–474.

35. Rissanen T, Voutilainen S, Nyyssönen K, Salonen JT. Lycopene, atherosclerosis, and coronary heart disease. Exp Biol Med (Maywood). 2002; 227:900–907.

36. Rissanen TH, Voutilainen S, Nyyssönen K, Salonen R, Kaplan GA, Salonen JT. Serum lycopene concentrations and carotid atherosclerosis: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2003; 77:133–138.

37. Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, Fontana J, Forman JD, Cher ML, Powell I, Pontes JE, Kucuk O. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007; 59:1–7.

38. Ylönen K, Alfthan G, Groop L, Saloranta C, Aro A, Virtanen SM. Dietary intakes and plasma concentrations of carotenoids and tocopherols in relation to glucose metabolism in subjects at high risk of type 2 diabetes: the Botnia Dietary Study. Am J Clin Nutr. 2003; 77:1434–1441.

39. Liu X, Qu D, He F, Lu Q, Wang J, Cai D. Effect of lycopene on the vascular endothelial function and expression of inflammatory agents in hyperhomocysteinemic rats. Asia Pac J Clin Nutr. 2007; 16:Suppl 1. 244–248.
40. Balestrieri ML, De Prisco R, Nicolaus B, Pari P, Moriello VS, Strazzullo G, Iorio EL, Servillo L, Balestrieri C. Lycopene in association with alpha-tocopherol or tomato lipophilic extracts enhances acylplatelet-activating factor biosynthesis in endothelial cells during oxidative stress. Free Radic Biol Med. 2004; 36:1058–1067.

41. Suganuma H, Inakuma T. Protective effect of dietary tomato against endothelial dysfunction in hypercholesterolemic mice. Biosci Biotechnol Biochem. 1999; 63:78–82.

42. Lowe GM, Booth LA, Young AJ, Bilton RF. Lycopene and beta-carotene protect against oxidative damage in HT29 cells at low concentrations but rapidly lose this capacity at higher doses. Free Radic Res. 1999; 30:141–151.

43. Yeh S, Hu M. Antioxidant and pro-oxidant effects of lycopene in comparison with beta-carotene on oxidant-induced damage in Hs68 cells. J Nutr Biochem. 2000; 11:548–554.

44. Piconi L, Quagliaro L, Assaloni R, Da Ros R, Maier A, Zuodar G, Ceriello A. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab Res Rev. 2006; 22:198–203.

45. Tamareille S, Mignen O, Capiod T, Rücker-Martin C, Feuvray D. High glucose-induced apoptosis through store-operated calcium entry and calcineurin in human umbilical vein endothelial cells. Cell Calcium. 2006; 39:47–55.

46. Kuki S, Imanishi T, Kobayashi K, Matsuo Y, Obana M, Akasaka T. Hyperglycemia accelerated endothelial progenitor cell senescence via the activation of p38 mitogen-activated protein kinase. Circ J. 2006; 70:1076–1081.

47. Seeger FH, Haendeler J, Walter DH, Rochwalsky U, Reinhold J, Urbich C, Rössig L, Corbaz A, Chvatchko Y, Zeiher AM, Dimmeler S. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation. 2005; 111:1184–1191.

48. Adams RH, Porras A, Alonso G, Jones M, Vintersten K, Panelli S, Valladares A, Perez L, Klein R, Nebreda AR. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol Cell. 2000; 6:109–116.

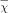 ± SD). Analysis of variance was used for group comparisons. Statistical analysis was performed using analysis of variance for mean comparison of multiple samples, LSD tests for paired comparison, and variance analysis of replicate measurements data for comparison of different experiment intervals. Absolute values, which were equal to differences in functional parameters between the lycopene plus HG group, were subjected to curve fitting analysis. The level of statistical significance for all analyses was set at α = 0.05.
± SD). Analysis of variance was used for group comparisons. Statistical analysis was performed using analysis of variance for mean comparison of multiple samples, LSD tests for paired comparison, and variance analysis of replicate measurements data for comparison of different experiment intervals. Absolute values, which were equal to differences in functional parameters between the lycopene plus HG group, were subjected to curve fitting analysis. The level of statistical significance for all analyses was set at α = 0.05.



 PDF
PDF ePub
ePub Citation
Citation Print
Print









 XML Download
XML Download