This article has been corrected. See "Erratum: Funding Acknowledgment" in Volume 6 on page 270.
Abstract
Schizandra chinensis Baillon is a traditional folk medicine plant that is used to treat and prevent several inflammatory diseases and cancer in Korea, but the underlying mechanisms involved in its anti-allergic activity are not fully understood. This study was designed to investigate mechanisms of anti-allergic activity of a Schizandra chinensis Baillon water extract (SCWE) in immunoglobulin E (IgE)-antigen complex-stimulated RBL2H3 cells and to assess whether gastric and intestinal digestion affects the anti-allergic properties of SCWE. Oxidative stress is an important consequence of the allergic inflammatory response. The antioxidant activities of SCWE increased in a concentration-dependent manner. RBL-2H3 cells were sensitized with monoclonal anti-dinitrophenol (DNP) specific IgE, treated with SCWE, and challenged with the antigen DNP-human serum albumin. SCWE inhibited β-hexosaminidase release and expression of interleukin (IL)-4, IL-13, and tumor necrosis factor-alpha (TNF-α) mRNA and protein in IgE-antigen complex-stimulated RBL2H3 cells. We found that digested SCWE fully maintained its antioxidant activity and anti-allergic activity against the IgE-antigen complex-induced activation of RBL-2H3 cells. SCWE may be useful for preventing allergic diseases, such as asthma. Thus, SCWE could be used as a natural functional ingredient for allergic diseases in the food and/or pharmaceutical industries.
In an anaphylactic type of allergy, binding of immunoglobulin E (IgE) and antigen to the high affinity IgE receptor (FcεRI) on the surface of mast cells and basophils induces the release of preformed intragranular mediators [1]. Mast cells and basophils play important roles initiating and perpetuating the inflammatory responses that mediate allergic reactions by secreting abundant levels of proinflammatory mediators such as histamine and several cytokines, including interleukin-4 (IL-4), IL-5, IL-13, and tumor necrosis factor (TNF)-α [2]. Mast cell and basophil activation is initiated when antigens with surface-bound IgE bind to FcεRI on the mast cell surface and induce degranulation and release of cytokines [3]. The rat basophilic leukemia cell line RBL-2H3, which expresses FcεRI, is widely used to study the molecular basis of mast cell activation [3]. This cell line has also been used to develop anti-allergy drugs that reduce allergic symptoms, including steroids that have anti-histamine actions and anti-inflammatory drugs that inhibit cytokine production [2,3]. However, the effectiveness of such drugs is limited by their side effects. These problems have led to increasing interest in traditional herbal medicines that have been used to treat allergic diseases. As a result, more and more studies examining the efficacy of natural extracts and compounds isolated from natural extracts to prevent and treat allergic disorders are being performed.
In the last few decades in Korea, China, and Japan, Schisandra chinensis Baillon (Korean name: Omija; English name: Chinese magnolia fruit) has been used by herbalists to regulate various pathophysiological conditions [4]. For example, it has been used to treat and prevent several chronic inflammatory diseases (e.g., asthma), hepatitis, and cancer [5] that may be the result of biomolecular damage due to free radicals and reactive oxygen species. However, the molecular mechanism by which S. chinensis Baillon counters antigen-mediated allergic diseases is not yet understood.
To elucidate this mechanism, we generated a S. chinensis Baillon water extract (SCWE) and tested its ability to inhibit degranulation (release of β-hexosaminidase) and allergy-related cytokine generation (IL-4, IL-13, and TNF-α) in RBL-2H3 cells stimulated with the dinitrophenyl (DNP)-specific IgE-antigen complex. We also determined whether gastric and intestinal digestion affects the anti-allergic effect of SCWE against the IgE-antigen complex-induced activation of RBL-2H3 cells. Oxidative stress is an important consequence of the inflammatory response in allergic diseases [6]. Thus, we also measured the antioxidant properties of SCWE and digested SCWE (DSCWE).
The following materials were purchased from the indicated commercial sources: PowerScript reverse transcriptase (Clontech, Palo Alto, CA, USA), oligo(dT)15 primers (Promega, Madison, WI, USA), GoTaq® Green Master Mix (Promega), rat TNF-α ELISA kit (eBioscience, San Diego, CA, USA), ECL chemiluminescence kit (BD Biosciences, San Jose, CA, USA), anti-IL-4 (mouse monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-IL-13 (mouse monoclonal antibody; Santa Cruz Biotechnology). Folin-Ciocalteu reagent, caffeic acid, monoclonal anti-DNP IgE, DNP-conjugated human serum albumin (DNP-HSA), and all other reagents used were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
S. chinensis Baillon (fruits of Omija) was purchased from an herbal shop in Chuncheon-si, and was cleaned, dried and ground to fine power before being extracted three times with 10.7 volumes of distilled water in a 60℃ shaking incubator for 24 h. The extract was centrifuged, and the supernatant was filtered under vacuum, concentrated in a rotary evaporator, and lyophilized.
The simulated gastric and intestinal juices were prepared as follows. Gastric fluid consisted of 2.0 g NaCl and 3.2 g pepsin dissolved in 900 mL water. The pH was adjusted to 1.2 and distilled water was added to make 1,000 mL. The pH of the gastric fluid was pH 1.2. The intestinal fluid consisted of 6.8 g KH2PO4 and 10.0 g pancreatin in 700 mL water containing 190 mL of 0.2 N NaOH. The pH was adjusted to 7.5 and distilled water was added to make 1,000 mL [7].
The digested sample solution was prepared by the methods of Shon et al. [7] and Ryu et al. [8]. Lyophilized SCWE (1.0 g) was placed in 250 mL gastric fluid and the mixture was incubated in a 37℃ shaking incubator for 1 h. The mixture was adjusted to pH 7.5 with 0.2 N NaOH before 1 L intestinal juice was added. Thus, the volumetric ratio of gastric fluid to intestinal fluid was 1:4. The mixture was incubated in a 37℃ shaking incubator for 6 h. The DSCWE was then placed at 100℃ in a water bath for 5 min and subsequently put on ice. The DSCWE was then centrifuged, and the supernatant was filtered under vacuum, concentrated in a rotary evaporator, and lyophilized.
The concentration of phenolics in SCWE and DSCWE was determined by a slight modification of the Folin and Denis method [9]. Five mL of SCWE or DSCWE (0, 100, 250, 500, and 1,000 µg/mL) was mixed with 0.5 mL of Folin-Ciocalteau phenol reagent. After 3 min, 1 mL of saturated Na2CO3 solution was added to the mixture. The reaction was kept in the dark for 1 h, and the absorbance of the mixture was measured at 700 nm. A standard curve was prepared with gallic acid [10]. The total phenol content of the extract was estimated by comparison with the standard curve.
DPPH radical scavenging activity of the SCWE and DSCWE was measured according to the procedure described by Choi et al. [11] with slight modifications. Different concentrations (0, 100, 250, 500, and 1,000 µg/mL) of SCWE, DSCWE, or vitamin C (180 µL) were mixed with 120 µL of an ethanol solution containing the DPPH radical (1.5 × 10-4 M). The mixture was shaken vigorously and allowed to stand in the dark at room temperature for 30 min. Reduction of the DPPH radicals was measured by an enzyme linked immunoassay (ELISA) reader (VERSA max microplate reader, Molecular Devices, Sunnyvale, CA, USA) at 517 nm. Radical scavenging activity was measured as the decrease in DPPH absorbance, and the inhibition percentage was calculated using the following equation:
Scavenging activity (%) = (1-Asample(517nm)/Acontrol (517nm)) × 100
RBL-2H3 cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (PEST) at 37℃ in a humidified incubator (5% CO2, 95% air). Cell viability was determined using the MTT [3-(3,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay according to the method of Chang et al. [4]. Briefly, RBL-2H3 cells were seeded on 96-well plates. After growth in DMEM containing 10% FBS and 1% PEST at 37℃ for 24 h, various concentration (0, 100, 250, 500, and 1,000 µg/mL) of SCWE or DSCWE were added to 96-well plates, and the RBL-2H3 cells were incubated for 24 h. MTT (5 mg/mL) was added to each culture well (one tenth of the original culture volume). After incubating for another 4 h, the precipitate formed was then dissolved in DMSO during shaking for 30 min. The extent of the reduction of MTT was quantified by measuring the absorbance at 570 nm in a microplate reader. The density of formazan formed in control cells was considered 100% viability.
The β-hexosaminidase release assay was performed as described previously [3] with slight modifications. RBL-2H3 cells (2 × 105 cells) in 24-well plates were preincubated with 0.5 µg/mL anti-DNP IgE for 12 h. The cells were then washed with Siraganian buffer (pH 7.2, 119 mM NaCl, 5 mM KCl, 0.4 mM MgCl2, 25 mM PIPES), incubated in Siraganian buffer containing 5.6 mM CaCl2 and 0.1% bovine serum albumin (BSA) for an additional 10 min, and exposed to 1 mL of 0, 100, 250, 500 and 1,000 µg/mL SCWE for 30 min. Thereafter, the cells were stimulated for 2 h with DNP-HSA (10 µg/mL), which activates RBL-2H3 cells into producing allergic reactions. The supernatant (20 µL) was mixed with an equal volume (20 µL) of substrate solution (2 mM p-nitrophenyl-N-acetyl-β-D-glucosaminide in 0.1 M sodium citrate buffer, pH 1.5), and the mixture was incubated for 1 h at 37℃. The reaction was terminated by adding 200 µL of stopping buffer (0.1 M Na2CO3/NaHCO3, pH 10.0). The absorbance at 405 nm was measured with a microplate reader. The SCWE or DSCWE-mediated inhibition of β-hexosaminidase release was expressed as the inhibition percentage and was calculated using the following formula:
Inhibition (%) = [1-T (405 nm)/C (405 nm)] × 100
where C is IgE (+) + DNP-HSA (+) + test sample (-) and T is IgE (+) + DNP-HSA (+) + test sample (+).
RBL-2H3 cells (2 × 105 cells) were primed with anti-DNP IgE (0.5 µg/mL) for 12 h and treated with SCWE or DSCWE (0, 100, 250, 500, and 1,000 µg/mL) for 30 min. The cells were then washed with Siraganian buffer and incubated in Siraganian buffer containing 5.6 mM CaCl2 and 0.1% BSA. The cells were stimulated with DNP-HSA (1 µg/mL) for 2 h. The cells were used for reverse transcription-polymerase chain reaction (RT-PCR) and Western blot analyses.
The supernatants (100 µL per well) of these cells were then transferred to 96-well ELISA plates and TNF-α concentrations were determined using a rat TNF-α ELISA kit (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions. The ability of SCWE or DSCWE to inhibit TNF-α production was calculated relative to the TNF-α production associated when vehicle rather than SCWE or DSCWE was used.
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad CA, USA) according to the manufacturer's instructions. The RNAs were then reverse-transcribed using a Superscript II kit (Invitrogen) with the oligo(dT)15 primer according to the manufacturer's recommendations. The PCR was performed with a GoTaq® Green Master Mix PCR kit (Promega) in 20 µL of total reaction mixture containing 1 µL of the RT-reaction mixture and 0.5 µL of each primer (forward and reverse, 15 µM). The PCR primers sets and the thermocycling parameters used are listed in Table 1. The PCR samples were analyzed electrophoretically on a 1.2% agarose gel containing 0.2 mg/mL ethidium bromide at 90 V for 1 h. The bands were visualized under UV light. GAPDH transcripts served as internal controls. mRNA band density was quantified using SigmaGel software (Jandel Scientific, San Rafael, CA, USA).
Western blotting was used to analyze IL-4 and IL-13 protein expression. The treated cells were lysed in lysis buffer containing 0.1 M EDTA, 0.1 mM NaCl, 0.1% Triton X-100, and protease inhibitor cocktail (Sigma-Aldrich). The protein content of the whole cell lysate was measured with a Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Whole cell lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene difluoride membranes (GE Healthcare, Buckinghamshire, UK). After blocking, the membranes were incubated first with mouse anti-rat IL-4 or mouse anti-rat IL-13 antibody (1:500) and then with horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody. The immunoreactive proteins were visualized using the ECL Western Blot Detection System (GE Healthcare) and the protein band densities were quantified using SigmaGel software.
Data are expressed as means±standard deviation, and are the average values of three to five values per experiment. Analyses were performed using SPSS software version 10.0 (SPSS, Chicago, IL, USA). The experiments were repeated at least twice to confirm the results. Analysis of variance was conducted, and Duncan's multiple-range tests were used to determine significant differences between groups. The level of statistical significance was set to P < 0.05.
The SCWE yield was 15.9±1.6%. It is important that it maintains its biological activity after passing through the human digestive tract for SCWE to have an effect on human health. Consequently, SCWE that was digested with simulated gastric and intestinal juices was prepared (named DSCWE). To investigate the effect of SCWE and DSCWE on cell viability, RBL-2H3 cells were exposed for 24 h to various concentrations of SCWE or DSCWE ranging from 0 to 1,000 µg/mL. As shown in Fig. 1A and 1B, RBL-2H3 cell viability was not affected by any of the SCWE or DSCWE concentrations.
To determine SCWE and DSCWE antioxidant activity, the total phenolics levels and DPPH radical scavenging activities of various concentrations of SCWE and DSCWE (0, 100, 250, 500, and 1,000 µg/mL) were determined (Fig. 1C-E). Total phenolic levels and DPPH radical scavenging activities of SCWE and DSCWE increased in a dose-dependent manner. Although DSCWE did not differ significantly from SCWE in total phenolic levels or DPPH radical scavenging activity, both had lower DPPH radical scavenging activity than that of ascorbic acid (Fig. 1E). The phenolic content of SCWE correlated significantly with its antioxidant capacity (r = 0.94, P < 0.0001).
To investigate the ability of SCWE and DSCWE to inhibit degranulation, we primed RBL-2H3 cells with anti-DNP IgE, treated them with SCWE or DSCWE, stimulated them with DNP-HSA, and measured β-hexosamididase release. Stimulation with the IgE-antigen complex induced the release of β-hexosaminidase, which was significantly inhibited by SCWE and DSCWE (100, 250, 500, and 1,000 µg/mL) (Fig. 2A and 2B). Inhibition of the β-hexosaminidase release by SCWE was not significantly different from that in DSCWE-treated cells.
IL-4, IL-13, and TNF-α play important roles in allergic diseases [3]. Therefore, we determined whether SCWE and DSCWE could inhibit IL-4, IL-13 and TNF-α mRNA expression in RBL-2H3 cells stimulated by the IgE-antigen complex (Fig. 3A and 3B). The IgE-antigen complex increased the accumulation of IL-4, IL-13, and TNF-α mRNA in RBL-2H3 cells, and this effect was significantly suppressed by treatment with SCWE or DSCWE (Fig. 3A and 3B). For example, treatment with 500 µg/mL SCWE suppressed the IgE-antigen complex-induced expression of IL-4 and IL-13 mRNA by 8.3- and 2.8-fold, respectively compared to those in untreated cells (Fig. 3A). Moreover, treatment with 500 µg/mL DSCWE reduced the IL-4 and IL-13 mRNA levels by 3.5- and 1.6-fold, respectively. Additionally, treatment with 500 µg/mL SCWE and DSCWE inhibited TNF-α mRNA expression by 50.0- and 2.3-fold, respectively. SCWE inhibited IL-4 and TNF-α mRNA production slightly more potently than that of DSCWE (Fig. 3A and 3B).
We also investigated the effect of SCWE or DSCWE treatment on IgE-antigen complex-induced IL-4, IL-13, and TNF-α protein production by Western blot analysis (IL-4 and IL-13) and ELISA (TNF-α). The Western blot results were analyzed using SigmaGel software (Figs. 4B, 4C, 5B and 5C). The data showed that stimulating with the IgE-antigen complex increased IL-4, IL-13, and TNF-α protein levels in RBL-2H3 cells, and that treatment with 250, 500, and 1,000 µg/mL SCWE reduced IL-4 and IL-13 protein levels (Fig. 4A-C). DSCWE at 500 and 1,000 µg/mL also reduced IL-4 and IL-13 protein levels (Fig. 5A-C). The inhibitory effect of SCWE was slightly more potent than that of DSCWE (Figs. 4A-5C). Moreover, SCWE or DSCWE treatment (100, 250, 500, and 1,000 µg/mL) led to a reduction in TNF-α protein levels, compared with that of untreated IgE-antigen complex-stimulated RBL-2H3 cells (Fig. 6A and 6B).
Mast cells and basophils play central roles in the immediate allergic reactions that are mediated by IgE and antigens [2]. These Th2-type reactions are precipitated when specific IgE receptors on the surface of mast cells or basophils bind multivalent allergens with surface-bound IgE. This, in turn, leads to the release of inflammatory mediators such as histamine and β-hexosaminidase, and cytokines such as IL-4, IL-13, and TNF-α [2].
Rat basophilic leukemia RBL-2H3 cells are mucosal mast cell-type cells and are frequently used to study the anti-allergenic activity of natural extracts [12,13]. When RBL-2H3 cells are primed with DNP-IgE, stimulation with antigen (DNP-HSA) induces them to rapidly degranulate and produce Th2 cytokines, including IL-4 and IL-13 [14]. As these responses closely mimic what occurs during the immediate allergic response, we selected the RBL-2H3 cell line, and IgE-antigen (DNP-HSA) complex-stimulated RBL-2H3 cells were used as the allergic cell model for our study.
S. chinensis Baillon is used as a medicinal plant in Asia [4]. In this study, we generated SCWE to examine its ability to suppress the IgE-antigen complex-induced allergic activation of RBL-2H3 cells. SCWE contains polyphenol compounds and it may provide protection against an allergic response. When phenolic compounds are consumed in the diet, the stomach enhances the release of phenolic compounds in natural extracts [15]. Phenolic compounds are absorbed from the stomach in a free form [16,17] and are extensively metabolized in the stomach and intestines [15]. Flavonoids, the main category of polyphenols, are absorbed from the small intestine. Subsequently, flavonoids are methylated, sulfated or glucuronidated as conjugates [18]. Anthocyanins are very sensitive to pH and are relatively unstable; thus, the color changed under different pH conditions (red in the acidic condition and purple in the neural condition). Glycosidic anthocyanins are abundant at low pH but anthocyanidins (aglycone) are exhibited in mildly acidic conditions [18]. Thus, some biological activities of phenolic compounds may be altered by changes in the stomach due to the acidic and neutral environments of the stomach and intestines, respectively. It is thus necessary to analyze the biological properties of their digestion products. It is unknown whether digested DSCWE retains its biological activity. We subjected SCWE to digestion using a simulated human digestive system to determine whether digestion affects the functional properties of SCWE. Simulated saliva and gastric juices as a model system has been used in previous studies [7,8], so we chose the same model system to test the effect of artificial digestive juice on antioxidant and anti-allergic activities of SCWE. We found that DSCWE fully maintained antioxidant and anti-allergic activities compared with those of SCWE as assessed by total phenolics levels, DPPH radical scavenging activity, β-hexosaminidase release, and measurements of allergy-related cytokine (IL-4, IL-13, and TNF-α) production. Our results suggest that the antioxidant and anti-allergic compounds in SCWE were not inactivated by gastric acid and intestinal juices.
Oxidative stress is an important consequence of the inflammatory response in allergic diseases, and oxidative stress is associated with a decrease in antioxidant activity in the respiratory tract [6]. Thus, we first examined the antioxidant activities of SCWE and DSCWE and the effect of both preparations on the secretion of β-hexosaminidase in IgE-antigen complex-stimulated RBL-2H3 cells. These two cellular properties may be mechanistically related, because allergic inflammation is believed to be driven by the reactive oxygen species (ROS) that are generated by macrophages, neutrophils, and mast cells in response to various factors. These ROS may then promote degranulation of mast cells, which can be determined by measuring the release of β-hexosaminidase, which is the granule marker of mast cells [3]. These observations suggest that compounds with antioxidant properties may be able to inhibit the mast cell degranulation that occurs during an allergic reaction. Many plants contain pharmacological compounds with antioxidant properties [19]. That such antioxidant plant compounds could have anti-allergy capability is supported by two recent studies showing that polyphenolic compounds from tea and coffee inhibit degranulation and histamine release from mast cells [12,20]. In our study, measurements of the total polyphenol levels and antioxidant activity of SCWE and DSCWE revealed that both increased in a concentration-dependent manner. This is consistent with a study showing that the total phenol content of fruit and plant extracts correlates well with their antioxidant capacities [21]. Notably, SCWE and DSCWE also markedly inhibited the increase in β-hexosaminidase release in IgE-antigen complex-stimulated RBL-2H3 cells. Thus, the antioxidant activity of the polyphenolic compounds in SCWE may be responsible for the ability of SCWE to inhibit β-hexosaminidase release.
We also examined the effects of SCWE and DSCWE on cytokine production of in IgE-antigen complex-stimulated RBL-2H3 cells. It is known that various cytokines, including the proinflammatory cytokine TNF-α and the Th2 cytokines such as IL-4, IL-5, and IL-13, play critical roles in allergic inflammation [2,22]. IL-4 is essential for IgE production [23] and promotes the switch from naive T cells to allergic type Th2 cells [24]. IL-13 affects many processes that contribute to an asthmatic phenotype, including inflammation, fibrosis, and mucus production. As a result, a number of experimental approaches have sought to neutralize the production of IL-13 as a novel therapeutic strategy for allergic asthma [25,26]. TNF-α is a potent inflammatory mediator that is mainly produced by activated various cell types in response to allergic pulmonary inflammation, including mast cells, macrophages, basophils, neutrophils, eosinophils, and epithelial cells. TNF-α is also synthesized and secreted by mast cells and basophils as a result of IgE and antigen challenge [27]. We found that SCWE and DSCWE inhibited upregulation of TNF-α, IL-4, and IL-13 mRNA and protein in IgE-antigen complex-stimulated RBL-2H3 cells. These results are consistent with the finding that anti-allergenic compounds such as thunberginol B and luteolin inhibit upregulation of TNF-α, IL-2, IL-4 and IL-13 mRNA after RBL-2H3 cells are stimulated with DNP-BSA [2]. We found that TNF-α protein levels did not decrease in a dose-dependent manner after SCWE or DSCWE treatment compared to that in untreated control cells, suggesting that TNF-α, a potent proinflammatory cytokine with immunoregulatory activities, responded strongly at low concentrations of SCWE or DSCWE. Quan et al. [28] reported that allergy-related cytokines such as IL-4 and IL-13 do not change in a dose-dependent manner following treatment with anti-allergic extract (Saururus chinensis ethanol extract) and that IL-4 and IL-13 levels decreased significantly at a doses of 10 and 100 mg/kg S. chinensis ethanol extract with similar levels. It is possible that some types of anti-allergic extracts and anti-allergy drugs may dose-dependently decrease allergy-related cytokines levels, whereas others may not.
In conclusion, we found that SCWE inhibited degranulation, transcription, and translation of allergy-related cytokines in IgE-antigen complex-stimulated RBL-2H3 cells, and that gastric and intestinal digestion did not significantly alter these properties. These results suggest that SCWE may be protective against allergic diseases by antigen-induced activation and that SCWE could be a beneficial food ingredient for preventing inflammatory diseases, such as allergic asthma. The artificial digestion system may use a useful model during the search for a therapeutic candidate against antigen-mediated allergic diseases before experimentation on animals or/and humans.
References
1. Itoh T, Ohguchi K, Iinuma M, Nozawa Y, Akao Y. Inhibitory effect of xanthones isolated from the pericarp of Garcinia mangostana L. on rat basophilic leukemia RBL-2H3 cell degranulation. Bioorg Med Chem. 2008; 16:4500–4508. PMID: 18328716.
2. Matsuda H, Wang Q, Matsuhira K, Nakamura S, Yuan D, Yoshikawa M. Inhibitory effects of thunberginols A and B isolated from Hydrangeae Dulcis Folium on mRNA expression of cytokines and on activation of activator protein-1 in RBL-2H3 cells. Phytomedicine. 2008; 15:177–184. PMID: 17950587.

3. Huang F, Yamaki K, Tong X, Fu L, Zhang R, Cai Y, Yanagisawa R, Inoue K, Takano H, Yoshino S. Inhibition of the antigen-induced activation of RBL-2H3 cells by sinomenine. Int Immunopharmacol. 2008; 8:502–507. PMID: 18279805.

4. Chang GT, Kang SK, Kim JH, Chung KH, Chang YC, Kim CH. Inhibitory effect of the Korean herbal medicine, Dae-Jo-Whan, on platelet-activating factor-induced platelet aggregation. J Ethnopharmacol. 2005; 102:430–439. PMID: 16125889.

5. Hancke JL, Burgos RA, Ahumada F. Schisandra chinensis (Turcz.) Baill. Fitoterapia. 1999; 70:451–471.
7. Shon MY, Choi SD, Kahng GG, Nam SH, Sung NJ. Antimutagenic, antioxidant and free radical scavenging activity of ethyl acetate extracts from white, yellow and red onions. Food Chem Toxicol. 2004; 42:659–666. PMID: 15019191.

8. Ryu JS, Hyun JW, Kim HW, Shim MJ, Kim BK. Effects of artificial digestive juice on the antitumor-immunity activity of protein-bound polysaccharide from Ganoderma lucidum. Yakhak Hoeji. 2000; 44:347–353.
9. Folin O, Denis W. A colorimetric method for the determination of phenols (and phenol derivatives) in urine. J Biol Chem. 1915; 22:305–308.

10. Mărghitaş LA, Stanciu OG, Dezmirean DS, Bobiş O, Popescu O, Bogdanov S, Campos MG. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009; 115:878–883.

11. Choi HJ, Chung MJ, Ham SS. Antiobese and hypocholesterolaemic effects of an Adenophora triphylla extract in HepG2 cells and high fat diet-induced obese mice. Food Chem. 2010; 119:437–444.
12. Yamashita K, Suzuki Y, Matsui T, Yoshimaru T, Yamaki M, Suzuki-Karasaki M, Hayakawa S, Shimizu K. Epigallocatechin gallate inhibits histamine release from rat basophilic leukemia (RBL-2H3) cells: role of tyrosine phosphorylation pathway. Biochem Biophys Res Commun. 2000; 274:603–608. PMID: 10924324.

13. Watanabe J, Shinmoto H, Tsushida T. Coumarin and flavone derivatives from estragon and thyme as inhibitors of chemical mediator release from RBL-2H3 Cells. Biosci Biotechnol Biochem. 2005; 69:1–6. PMID: 15665459.

14. Fukao T, Terauchi Y, Kadowaki T, Koyasu S. Role of phosphoinositide 3-kinase signaling in mast cells: new insights from knockout mouse studies. J Mol Med (Berl). 2003; 81:524–535. PMID: 12928787.

15. Scalbert A, Morand C, Manach C, Rémésy C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed Pharmacother. 2002; 56:276–282. PMID: 12224598.

16. Zhao Z, Egashira Y, Sanada H. Ferulic acid is quickly absorbed from rat stomach as the free form and then conjugated mainly in liver. J Nutr. 2004; 134:3083–3088. PMID: 15514279.

17. Lafay S, Gil-Izquierdo A, Manach C, Morand C, Besson C, Scalbert A. Chlorogenic acid is absorbed in its intact form in the stomach of rats. J Nutr. 2006; 136:1192–1197. PMID: 16614403.

18. Aura AM. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem Rev. 2008; 7:407–429.

19. Nishikawa H, Kitani S. Tea catechins have dual effect on mast cell degranulation induced by compound 48/80. Int Immunopharmacol. 2008; 8:1207–1215. PMID: 18602066.

20. Ramalakshmi K, Jagan Mohan Rao L, Takano-Ishikawa Y, Goto M. Bioactivities of low-grade green coffee and spent coffee in different in vitro model systems. Food Chem. 2009; 115:79–85.

21. Wijngaard HH, Rössle C, Brunton N. A survey of Irish fruit and vegetable waste and by-products as a source of polyphenolic antioxidants. Food Chem. 2009; 116:202–207.

22. Lee SH, Bae EA, Park EK, Shin YW, Baek NI, Han EJ, Chung HG, Kim DH. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps in IgE-induced hypersensitivity. Int Immunopharmacol. 2007; 7:1678–1684. PMID: 17996677.

23. Kühn R, Rajewsky K, Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991; 254:707–710. PMID: 1948049.

24. Hines C. The diverse effects of mast cell mediators. Clin Rev Allergy Immunol. 2002; 22:149–160. PMID: 11975420.

25. Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998; 282:2261–2263. PMID: 9856950.

26. Yang G, Li L, Volk A, Emmell E, Petley T, Giles-Komar J, Rafferty P, Lakshminarayanan M, Griswold DE, Bugelski PJ, Das AM. Therapeutic dosing with anti-interleukin-13 monoclonal antibody inhibits asthma progression in mice. J Pharmacol Exp Ther. 2005; 313:8–15. PMID: 15644434.

27. Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990; 346:274–276. PMID: 2374592.
28. Quan Z, Lee YJ, Yang JH, Lu Y, Li Y, Lee YK, Jin M, Kim JY, Choi JH, Son JK, Chang HW. Ethanol extracts of Saururus chinensis suppress ovalbumin-sensitization airway inflammation. J Ethnopharmacol. 2010; 132:143–149. PMID: 20699114.

Figure 1
RBL-2H3 cell toxicity to the Schisandra chinensis water extract (SCWE) and digested SCWE (DSCWE) and their antioxidant effects in a cell-free system. A and B, Cytotoxicity. After a 24 h treatment with various SCWE or DSCWE concentrations (0, 100, 250, 500, and 1,000 µg/mL), RBL-2H3 cell viability was determined by the MTT assay. C and D, Total phenol content. The Folin-Ciocalteu method was used. E, DPPH radical scavenging activity. Various concentrations of SCWE or DSCWE (0, 100, 250, 500, and 1,000 µg/mL) were mixed with the DPPH solution and incubated at 37℃, and the absorbance change at 517 nm was then determined. The reference compound was ascorbic acid. Means with different letters (a-e) differ significantly from each other (P < 0.05), as determined by Duncan's multiple range test (n = 4). Cell viability results were not significantly different (P > 0.05).
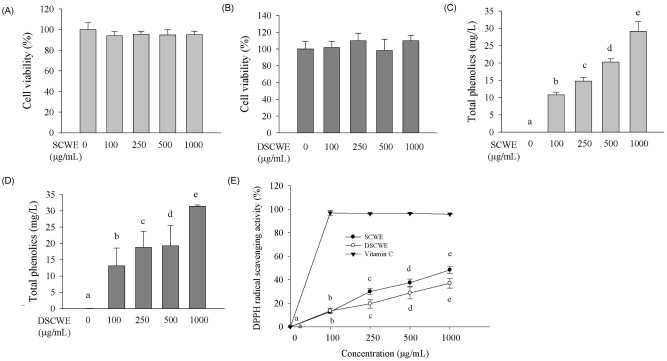
Figure 2
Effect of Schisandra chinensis water extract (SCWE) and digested SCWE (DSCWE) on β-hexosaminidase release in RBL-2H3 cells stimulated by the anti-DNP IgE-antigen (DNP-HSA) complex. RBL-2H3 cells (2 × 105 cells) in 24-well plates were preincubated with 0.5 µg/mL anti-DNP IgE for 12 h, washed with Siraganian buffer, incubated in Siraganian buffer containing 5.6 mM CaCl2 and 0.1% BSA for 10 min, and then treated with 1 mL of SCWE or DSCWE (0, 100, 250, 500, and 1,000 µg/mL) for 30 min. Cells were stimulated for 2 h with DNP-HSA (10 µg/mL) to activate the cells and evoke an allergic reaction. β-hexosaminidase secretion into the supernatant was then measured. Means with different letters (a-f) differ significantly from each other (P < 0.05), as determined by Duncan's multiple range test (n = 4). control, C.
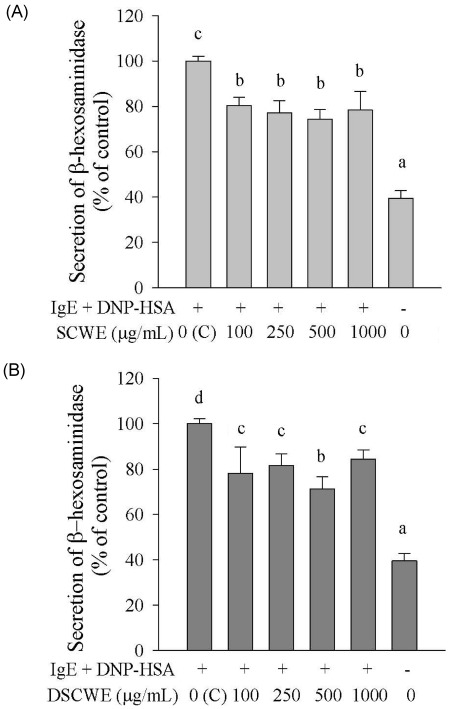
Figure 3
Effects of Schisandra chinensis water extract (SCWE) and digested SCWE (DSCWE) on the expression of interleukin (IL)-4, IL-13 and tumor necrosis factor (TNF)-α mRNA in RBL-2H3 cells stimulated with the IgE-antigen (DNP-HSA) complex. RBL-2H3 cells were preincubated with anti-DNP IgE (0.5 µg/mL) for 12 h, treated with various concentration (0, 100, 250, 500, and 1,000 µg/mL) of SCWE or DSCWE for 30 min, and stimulated with DNP-HSA (1 µg/mL) for 2 h. The cells were then washed, lysed, and subjected to reverse transcription-polymerase chain reaction (RT-PCR). The IL-4, IL-13, and TNF-α mRNA levels in each sample were normalized to GAPDH levels. mRNA band density was quantified using SigmaGel software, and the group data were averaged and plotted. Means for each gene with different letters (a-e) differ significantly from each other (P < 0.05), as determined by Duncan's multiple range test (n = 4). Control, C.
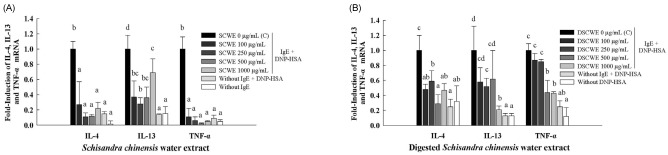
Figure 4
Effects of Schisandra chinensis water extract (SCWE) on interleukin (IL)-4 and IL-13 protein production in RBL-2H3 cells stimulated with the IgE-antigen (DNP-HSA) complex. The cells were primed with anti-DNP IgE (0.5 µg/mL) for 12 h, treated with various concentrations (0, 100, 250, 500, and 1,000 µg/mL) of SCWE for 30 min, stimulated with DNP-HSA (1 µg/mL) for 2 h, washed, lysed, and subjected to Western blot analysis. IL-4 and IL-13 protein levels in each sample were normalized to α-tubulin levels. Protein band density was quantified using SigmaGel software, and the group data were averaged and plotted. Means for each protein with different letters (a-f) differ significantly from each other (P < 0.05), as determined by Duncan's multiple range test (n = 4). Control, C.
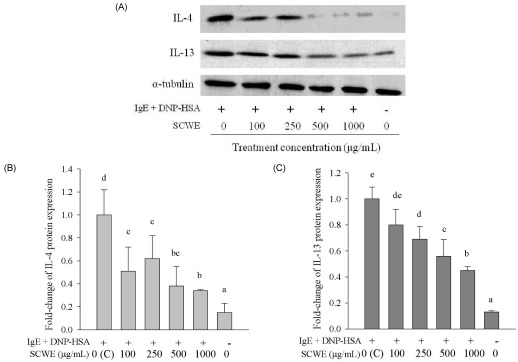
Figure 5
Effects of digested Schisandra chinensis water extract (DSCWE) on interleukin (IL)-4 and IL-13 protein production in RBL-2H3 cells stimulated with the IgE-antigen (DNP-HSA) complex. The cells were primed with anti-DNP IgE (0.5 µg/mL) for 12 h, treated with various concentrations (0, 100, 250, 500, and 1,000 µg/mL) of DSCWE for 30 min, stimulated with DNP-HSA (1 µg/mL) for 2 h, washed, lysed, and subjected to Western blot analysis. IL-4 and IL-13 protein levels in each sample were normalized to α-tubulin levels. Protein band density was quantified using SigmaGel software, and the group data were averaged and plotted. Means for each protein with different letters (a-d) differ significantly from each other (P < 0.05), as determined by Duncan's multiple range test (n = 4). Control, C.
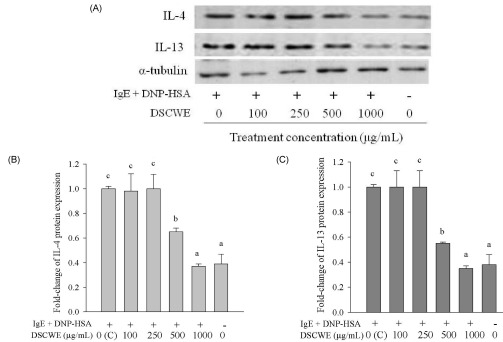
Figure 6
Effects of Schisandra chinensis water extract (SCWE) and digested SCWE (DSCWE) on the tumor necrosis factor (TNF)-α protein production in RBL-2H3 cells stimulated with the IgE-antigen (DNP-HSA) complex. RBL-2H3 cells were preincubated with anti-DNP IgE (0.5 µg/mL) for 12 h, treated with various concentrations (0, 100, 250, 500, and 1,000 µg/mL) of SCWE or DSCWE for 30 min, and stimulated with DNP-HSA (1 µg/mL) for 2 h. The supernatant was subjected to enzyme-linked immunoabsorbent assay (ELISA). Means with different letters (a-e) differ significantly from each other (P < 0.05), as determined by Duncan's multiple range test (n = 4). Control, C.

Table 1
Polymerase chain reaction primer sequences and thermocycling parameters
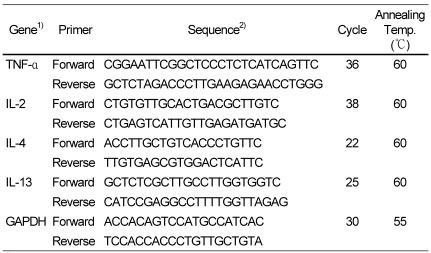
1)TNF-α, tumor necrosis factor-α; IL-2, interleukin-2; IL-4, interleukin-4; IL-13, interleukin-13; GAPDH, glyceraldehyde-3-phosphate dehydrogenase
2)Primers are shown 5 → 3. Each cycle consisted of denaturation at 94℃ for 4 min, annealing at each temperature for 30 s, and extension at 72℃ for 5 min.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download