Abstract
Glycyrrhiza uralensis (or licorice) is a widely used Oriental herbal medicine from which the phenylflavonoids dehydroglyasperin C (DGC), dehydroglyasperin D (DGD), and isoangustone A (IsoA) are derived. The purpose of the present study was to evaluate the antioxidant properties of DGC, DGD, and IsoA. The three compounds showed strong ferric reducing activities and effectively scavenged DPPH, ABTS+, and singlet oxygen radicals. Among the three compounds tested, DGC showed the highest free radical scavenging capacity in human hepatoma HepG2 cells as assessed by oxidant-sensitive fluorescent dyes dichlorofluorescein diacetate and dihydroethidium bromide. In addition, all three compounds effectively suppressed lipid peroxidation in rat tissues as well as H2O2-induced ROS production in hepatoma cells. This study demonstrates that among the three phenylflavonoids isolated from licorice, DGC possesses the most potent antioxidant activity, suggesting it has protective effects against chronic diseases caused by reactive oxygen species as well as potential as an antioxidant food additive.
Oxidative stress occurs as a result of an imbalance between reactive oxygen species (ROS) formation and the capacity of antioxidant defenses [1,2]. ROS include superoxide anions, singlet oxygen, nitric oxide, peroxynitrite, hydrogen peroxide, and hydroxyl radicals, all of which can induce chronic diseases such as cancer, aging, as well as neuro-degenerative and cardiovascular diseases by damaging lipids, DNA, and proteins [1,3].
Unlike their natural counterparts, synthetic antioxidants have been found to cause side effects such as toxicity, cell damage, inflammation, and atherosclerosis in animals and humans. In recent years, it has been well established that certain plant-derived compounds or phytochemicals, including phenols and alkaloids, possess therapeutic antioxidant efficacy and thus could be utilized as anti-inflammatory, anti-allergic, hepatoprotective, anti-thrombotic, anti-viral, anti-malaria, anti-atherosclerotic, and anti-carcinogenic agents [4,5].
Glycyrrhiza uralensis (or licorice) is a widely used Oriental herbal medicine as well as food additive [6]. Licorice has been reported to have therapeutic effects on malaria, cardiovascular disease, peptic ulcers, hepatitis C, and pulmonary and skin diseases [7]. Among the constituents of licorice, glycycoumarin (G. uralensis), glabridin (G. glabra), and licochalcone A (G. inflate) are all known to function as indicators [8]. Dehydroglyasperin C (DGC) and dehydroglyasperin D (DGD) in licorice are characterized by increased PPAR-gamma ligand-binding as well as anti-inflammatory activities [6,9], whereas isoangustone A (Iso A), another bioactive constituent, displays anti-bacterial [10] and anti-inflammatory activities in renal mesangial cells [11], along with apoptotic effects in prostate cancer cells [12,13]. It has also been reported that DGC plays an important role in cancer prevention by inducing detoxifying enzymes via activation of Nrf2/ARE [14]. Despite ample evidence that the ability of phytochemicals to induce the Nrf2 signaling pathway is closely related to their antioxidant activities in most cases, there is only limited information on the antioxidant activities of phenylflavonoids such as DGC in licorice. Thus, the present study was designed in order to compare the antioxidant potentials of the phenylflavonoids dehydroglyasperin C (DGC), dehydroglyasperin D (DGD), and isoangustone A (IsoA) isolated from licorice.
2,4,6-tripyridyl-s-triazine (TPTZ), ferric chloride, 7'-dichlorofluorescein diacetate (DCFDA), dihydroethidium (DHE), and 2,6-di-tert-butyl-4-methylphenol (BHT) were purchased from Sigma (St. Louis, MO, USA). Trichloroacetic acid was supplied from Kanto Chemical Co. (Japan). All other reagents used were of ACS grade. Dehydroglyasperin C (DGC), dehydroglyasperin D (DGD), and isoangustone A (Iso A) (Fig. 1) were supplied by Dr. Lim at Hallym University Regional Innovation Center (RIC) in Chuncheon, as previously reported [15].
The FRAP of the sample was measured according to the redox-linked colorimetric method [16]. In this assay, ferric-2,4,6-tripyridyl-s-triazine (Fe (III)-TPTZ) complex is reduced to ferrous ion, which is blue-colored. Briefly, 30 µL of FRAP working reagent consisting of 300 mM acetate buffer (pH 3.6), 10 mM Fe (III)-TPTZ in 40 mM HCl, and 20 mM FeCl3·H2O at a ratio of 10:1:1 was mixed with 7 µL of sample or standard, followed by addition to 170 µL of distilled water in a 96-well microplate. After incubation for 10 min at room temperature, the absorbance at 593 nm was measured using an ELISA reader (Tecan Sunrise microplate reader, ReTiSoft Inc. Mississauga, Ontario, Canada). α-Tocopherol and trolox were used as positive controls.
DPPH radical scavenging activity was assayed by following the previous method with minor modifications [17]. Briefly, 50 µL of each sample was added to 200 µL of 200 µM DPPH methanolic solution in a 96-well microplate. After shaking slightly, the plate was incubated at 37℃ for 30 min, after which the absorbance was measured at 515 nm. Dimethyl sulfoxide (DMSO) and methanol were used as negative controls, whereas α-tocopherol and trolox were used as positive controls.
Decolorization of ABTS·+ was determined according to a previously modified method [18]. ABTS·+ was generated through a stoichiometrically reaction involving 5 mL of 7 mM ABTS and 80 µL of 2.45 mM potassium persulfate in the dark at room temperature for 16 h. Dilution was carried out with ethanol in order to obtain the proper absorbance of 0.7 ± 0.02 at a wavelength of 734 nm. Then, 90 µL of ABTS·+ solution was added to a 96-well plate containing of 10 µL of sample. Absorbance was detected at 734 nm after incubation at room temperature for 5 min.
To assess the ability of sample to scavenge singlet oxygen (1O2), the imidazole-RNO method was employed, in which 1O2 is photogenerated from rose bengal (RB, a well known type II photosensitizer) [19]. The 1O2-mediated bleaching of RNO via imidazole oxidation was monitored spectrophotometrically at 440 nm in reaction mixtures consisting of RB (3 µM), imidazole (5 mM), and RNO (4 µM) in 20 mM Tris-succinate buffer (pH 6.5). The sample mixtures were then allowed to pass through a 6-mm Plexiglass and UV-35 filter from a 150 W Halogen-lamp (Osram, Augsburg, Germany) (white light (l > 350 nm)) for 3 min at 25℃. L-ascorbic acid was used as a positive control.
All cell culture reagents and fetal bovine serum (FBS) were obtained from Hyclone (Logan, KS, USA). HepG2 cells were from the Korean Cell Line Bank. HepG2 cells were fed Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS and 100 units/mL of penicillin/streptomycin at 37℃ in 5% CO2. Cells were incubated normally for 3 days in a humidified incubator and generally sub-cultured at a ratio of 1:4.
Intracellular reactive oxygen species (ROS) production was evaluated according to a previously reported method [20]. Briefly, HepG2 cells were seeded onto a 96-well black-bottom plate and cultured for 24 h. After adaptation, cells were stimulated by different concentrations of sample for another 24 h. Stimulated cells were then incubated with 50 µM dichlorofluorescein-diacetate (DCFDA) for 30 min, followed by 500 µM hydrogen peroxide diluted in phosphate-buffered saline (PBS) containing 0.5% FBS for 1 h. Fluorescence was measured at 0 and 30 min at an excitation wavelength of 485 nm and emission wavelength of 535 nm on a TECAN microplate reader (Grodig, Austria). The calculation formula was [(F40min - F0min)/F0min] × 100.
DHE assay was conducted according to the following procedure [21]. Fluorescent dye was used as a molecular probe in order to measure the generation of superoxide anion. The experimental procedure was identical to that of the DCF assay. The fluorescence probe used was 10 µM DHE, and fluorescence was measured at 0 and 30 min at an excitation wavelength of 540 nm and emission wavelength of 595 nm on a TECAN microplate reader (Grodig, Austria). The calculation formula was [(F30min - F0min) / F0min] × 100.
Lipid peroxidation assay was carried out by following a previously described method [22]. Rats were anesthetized with diethyl ether and sacrificed by exsanguination. The perfused liver was isolated and homogenized with isotonic PBS (nine parts) using a homogenizer at 4℃. The homogenate was then centrifuged at 10,000 × g for 5 min, after which the supernatant was used in an in vitro lipid peroxidation assay. Briefly, various concentrations of sample were mixed with 1 mL of 0.15 M potassium chloride and 0.5 mL of rat liver homogenate. Peroxidation was initiated by addition of 100 µL of 0.4 mM ferric chloride. After incubation at 37℃ for 30 min, the reaction was stopped by addition of 2 mL of ice-cold 0.25 M HCl containing 15% TCA, 0.38% TBA, and 0.5% BHT. The mixture was then heated at 90℃ for 30 min, cooled, and centrifuged at 10,000 × g for 5 min, after which the supernatant was read at 532 nm. The percentage of lipid peroxidation inhibitory activity was calculated as follows: lipid peroxidation rate = 1 - (sample OD / blank OD) × 100. A control without added sample was also run simultaneously. Briefly, rat brain and kidney were homogenized with a Polytron in 10 mL of ice-cold Tris-HCl buffer (20 mM, pH 7.4) [23]. The homogenate was then centrifuged at 24,795 × g for 15 min, after which the supernatants (1 mL) were incubated with different concentrations of sample in the presence of 10 µM FeSO4 and 0.1 mM ascorbic acid at 37℃ for 1 h. Reactions were terminated by addition of 1.0 mL of TCA (28%) and 1.5 mL of TBA (1%). The mixture was then heated at 90℃ for 15 min, cooled to room temperature, and centrifuged at 4,696 × g for 15 min, after which the color of the MDA-TBA complex in the supernatant was read at 532 nm using a spectrophotometer. BHT was used as a positive control. The inhibition ratio (%) was calculated as follows: Inhibition ratio (%) = (A - Al) / A × 100, where A is absorbance of the control and Al is absorbance of the test sample.
The highest FRAP values were obtained for DGC (1,169 ± 43 µM) and DGD (1,135 ± 16 µM) at a concentration of 1 mM, followed by IsoA (337 ± 46 µM). In the FRAP assay, DGC dose-dependently exhibited strong antioxidant activity, and a similar level of activity was observed for DGD. IsoA also exhibited increased FRAP at a concentration between 0.2-1 mM, showing 3-5 fold weaker activity compared to that of DGC or DGD. Therefore, the reducing power of IsoA was the lowest among the compounds tested (Table 1).
The antioxidant activities of DGC, DGD, and IsoA are shown in Table 2 as percentages of DPPH radical scavenging activity. The three compounds induced significant increases in free radical scavenging compared to control (P < 0.05). In line with the FRAP values, DGC and DGD exhibited strong reducing potentials as indicated by their high DPPH scavenging activity. DGC showed the highest free radical scavenging activities of 52.7, 95.4, and 95.9% at concentrations of 0.2, 0.5, and 1 mM, respectively, whereas the average DPPH radical scavenging activities of DGD and Iso A were 33.2, 82.4, and 91.4% and 31.6, 57.3, and 80.0%, respectively. The IC50 value for the DPPH scavenging activity of DGC was 0.205 ± 0.005 mM, which was lower than those of DGD and IsoA (0.309 ± 0.002 and 0.418 ± 0.015 mM, respectively).
In the ABTS+ radical scavenging assay, all three compounds exhibited dose-dependent effects as well as higher antioxidant activities compared to the positive controls α-trocopherol and trolox (Table 2). Specifically, the ABTS+ radical scavenging activities of DGC, DGD, and IsoA were 123 ± 2.2, 93 ± 0.6, and 93 ± 0.8%, respectively, whereas those of α-tocopherol and trolox were 81 ± 6.9 and 84 ± 3.2%, respectively. These results indicate that these three compounds are the major constituents responsible for the antioxidant activity of licorice. The IC50 value for the ABTS+radical scavenging activity of DGC was 0.465 ± 0.081 mM, which was lower than those of DGD and IsoA (0.635 ± 0.035 and 0.655 ± 0.042 mM, respectively).
Contrary to the data in the FRAP and DPPH assays, the antioxidant activity of IsoA (124 ± 2.3%) at the highest concentration was comparable with that of DGD (125 ± 1.3%). However, the overall order of activities was similar to those observed in the FRAP and DPPH assays.
To investigate the singlet oxygen scavenging cavenging activities of DGC, DGD, and IsoA, an imidazole-RNO bleaching test was performed (Fig. 2). Among the compounds tested, DGC showed the strongest singlet oxygen quenching effect. Both DGC and DGD at a concentration of 100 µM scavenged singlet oxygen at rates of 34.5 ± 2.8 and 7.9 ± 2.3% respectively, whereas IsoA did not show any significant activity (3.3 ± 1.8%). However, the singlet oxygen quenching activities of the tested phenylflavonoids were higher than that of the positive control ascorbic acid (5.9 ± 1.1% at 100 µM).
We further tested the inhibitory effects of the licorice-derived phenylflavonoids on ROS production using the oxidant-sensitive fluorescent dyes DCFDA and DHE. As shown in Fig. 3A, H2O2-induced ROS production was significantly suppressed by DGC, DGD, and IsoA. Intracellular ROS generation increased by about 6-fold upon addition of H2O2, whereas it clearly decreased upon treatment with 10 µM DGC, DGD, or IsoA. Further, intracellular ROS levels induced by H2O2 were reduced by DGC, DGD, and IsoA by 84.6, 52.9, and 52.8%, respectively. Production of superoxide anion induced by H2O2 was also inhibited by the three flavonoids in a dose-dependent manner as evaluated by DHE assay. Similar to the other assays, the antioxidant activity of DGC was most prominent. As shown in Fig. 3B, the H2O2-induced increase in intracellular ROS level (500 µM) was reduced to that of the negative control by 10 µM DGC.
The inhibitory effects of the phenylflavonoids on ferric chloride-driven lipid peroxidation were evaluated by measuring TBARS levels in homogenates of rat tissues, such as the liver (Fig. 4A), brain (Fig. 4B), and kidney (Fig. 4C). Tissue lipid peroxidation due to ferric chloride was significantly inhibited by all three phenylflavonoids in a dose-dependent manner. For instance, lipid peroxidation in the kidney homogenate induced by ferric chloride treatment was effectively suppressed by all three flavonoids. The levels of TBARS in the kidney homogenates treated with ferric chloride plus 0.5 mM DGC, DGD, or IsoA were 34.6 ± 0.9, 32.7 ± 0.4, or 39.3 ± 0.8% of that of tissue homogenate treated with ferric chloride alone, respectively. The inhibitory effects of the licorice-derived flavonoids on lipid peroxidation were comparable with that of BHT, which showed a TBARS level of 34.5 ± 0.8% compared to control treated with FeCl3 alone. In accordance with the inhibitory effects of the compounds on lipid peroxidation in the brain and kidney, inhibition of lipid peroxidation in the liver due to DGC was similar or slightly more potent than that induced by DGD or IsoA.
It is well known that ROS are over-produced under various stressful conditions, which promotes the development of chronic diseases such as cancer, diabetes, cardiovascular diseases, and hypertension [1,3]. Therefore, it is important to identify phytochemicals with antioxidant activity that could be used to prevent and treat ROS-associated chronic diseases [24-26].
Numerous plant constituents have been reported to have antioxidant activities. For instance, polyphenolics such as EGCG, resveratrol, and quercetin are typical examples that have been reported to possess antioxidant and radical scavenging properties [27,28]. Polyphenols, which are abundant in plants, fruits, and vegetables, have drawn much attraction due to their protective effects against cancer, cardiovascular decline, and cognitive function and memory impairment [29,30].
Licorice has been widely used as a flavoring and sweetening agent in tobacco products, chewing gum, candy, toothpaste, and beverages, and it has long been prescribed as a treatment in Oriental herbal medicine. Licorice root is also recognized by the National Cancer Institute as exerting cancer chemopreventive effects [31,32]. However, the chronic consumption of a large-quantity of licorice, which contains glycyrrhizin (GL), has been reported to result in severe hypertension, hypokalemia, and other signs of mineralocorticoid excess [33]. This can be attributed to glycyrrhetinic acid (GA), which is produced by the metabolism of GL by gut microorganisms, being a well-known inhibitor of 11β-hydroxysteroid dehydrogenase type 2 [34]. As licorice has been consumed by humans for several thousand years and is known to be safe for human consumption (except for its hypertension-inducing effects), it is worthwhile to determine whether or not licorice extracts lacking glycyrrhizin still exert any physiologically beneficial effect. To answer this, Seon and colleagues previously demonstrated that hexane/ethanol extract of G. uralensis (HEGU) contains no detectable amounts of GL and suppresses doxorubicin-induced apoptosis in H9c2 rat cardiac myoblasts. In addition, it was found that HEGU reduces then umbers of viable HT-29 human colon, MDA-MB-231 human breast, and DU145 human prostate cancer cells [12]. Licorice root contains triterpenesaponins, flavonoids, isoflavonoids and chalcones, coumarins, stilbenoids, as well as miscellaneous compounds as biologically active components [15,35]. Recently, Lee and colleagues attempted to isolate antioxidant compounds from licorice using ABTS assay and identified DGC, DGD, and IsoA as major antioxidants [15]. However, the antioxidant activities of these three compounds have not been comparatively studied until now. Therefore, the main objective of the current study was to compare the antioxidant activities of DGC, DGD, and IsoA, which are phenylflavonoids isolated from licorice. This work demonstrated, for the first time, the radical scavenging and cytoprotective effects of these compounds. In particular, we found that DGC is the most potent antioxidant of all three tested compounds, suggesting its potential as a preventive agent against ROS-related diseases.
As shown by the DPPH, FRAP, and ABTS+ radical scavenging assays, the antioxidant activities of the phenylflavonoids isolated from licorice were clearly dose-dependent. As an explanation, we speculate that phenolic hydroxyl groups within the compounds joined to form a ring structure is responsible for antioxidant activity [36].
To elucidate the protective activities of DGC, DGD, and IsoA against ROS-induced cellular damage, DCFH-DA and DHE assays were also performed. When applied individually, the three compounds prevented H2O2-induced cell death without any apparent cytotoxicity (data not shown). H2O2 alone increased intracellular ROS levels in a hepatoma cell line, whereas ROS production was significantly decreased upon co-treatment with each compound. Similar to the other in vitro assays, the ROS inhibitory effect of DGC was the most potent as the ROS level remained low between 0 and 10 µM DGC treatment.
Based on our results, the degree of lipid peroxidation can be used as a biomarker of the ability of natural compounds to prevent oxidative deterioration of lipids. We observed increased formation of lipid peroxides in FeCl3-treated animal tissues, including liver, brain, and kidney tissues, and found that pretreatment of cells with each compound resulted in reduced formation of lipid peroxides. Namely, cellular damage due to oxidative stress was much lower in the compound-treated cells. In addition, the protective effects of DGC, DGD, and IsoA against lipid peroxide formation were similar, with liver tissue demonstrating the highest inhibition of lipid peroxidation compared to other tissues.
Taken together, this study demonstrated that DGC, DGD, and IsoA isolated from licorice have significant antioxidant activities, and DGC has the most prominent antioxidant activity. Although the number and location of hydroxyl groups in DGC may be responsible for its antioxidant activity, the precise mechanism by which DGC exerts its strong antioxidant effects remains to be elucidated. In addition, the in vivo efficacy and bioavailability of the compound should be determined in order to more accurately predict its clinical utility.
Figures and Tables
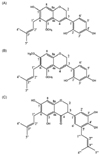 | Fig. 1Molecular structures of dehydroglyasperin C (A), dehydroglyasperin D (B), and Isoangustone A (C). |
 | Fig. 2
Singlet oxygen quenching capacities of increasing concentrations of DGC, DGD, IsoA, and ascorbic acid. Results are expressed as the means ± SD of three independent experiments. Means without a common letter differ, P < 0.05. |
 | Fig. 3
Inhibition of intracellular reactive oxygen species (ROS) generation by three phenylflavonoids isolated from licorice in HepG2 cells. ROS of a human hepatoma cell line were stained with DCFDA (A) and DHE (B). Results are expressed as the means ± SD of three independent experiments. Means without a common letter differ, P < 0.05. |
References
1. Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010. 4:118–126.

2. Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med. 2008. 8:63.

3. Halliwell B, Aeschbach R, Loliger J, Aruoma OI. The characterization of antioxidants. Food Chem Toxicol. 1995. 33:601–617.

4. Lopez-Varela S, Gonzalez-Gross M, Marcos A. Functional foods and the immune system: a review. Eur J Clin Nutr. 2002. 56:Suppl 3. S29–S33.

5. Veerapur VP, Prabhakar KR, Parihar VK, Kandadi MR, Ramakrishana S, Mishra B, Satish Rao BS, Srinivasan KK, Priyadarsini KI, Unnikrishnan MK. Ficus racemosa stem bark extract: apotent antioxidant and a probable natural radioprotector. Evid Based Complement Alternat Med. 2009. 6:317–324.

6. Mae T, Kishida H, Nishiyama T, Tsukagawa M, Konishi E, Kuroda M, Mimaki Y, Sashida Y, Takahashi K, Kawada T, Nakagawa K, Kitahara M. A licorice ethanolic extract with peroxisome proliferator-activated receptor-gamma ligand-binding activity affects diabetes in KK-Ay mice, abdominal obesity in diet-induced obese C57BL mice and hypertension in spontaneously hypertensive rats. J Nutr. 2003. 133:3369–3377.

7. Fukai T, Marumo A, Kaitou K, Kanda T, Terada S, Nomura T. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002. 71:1449–1463.

8. Kondo K, Shiba M, Yamaji H, Morota T, Zhengmin C, Huixia P, Shoyama Y. Species identification of licorice using nrDNA and cpDNA genetic markers. Biol Pharm Bull. 2007. 30:1497–1502.

9. Kuroda M, Mimaki Y, Sashida Y, Mae T, Kishida H, Nishiyama T, Tsukagawa M, Konishi E, Takahashi K, Kawada T, Nakagawa K, Kitahara M. Phenolics with PPAR-gamma ligand-binding activity obtained from licorice (Glycyrrhiza uralensis roots) and ameliorative effects of glycyrin on genetically diabetic KK-A(y) mice. Bioorg Med Chem Lett. 2003. 13:4267–4272.

10. Hatano T, Shintani Y, Aga Y, Shiota S, Tsuchiya T, Yoshida T. Phenolic constituents of licorice. VIII. Structures of glicophenone and glicoisoflavanone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus. Chem Pharm Bull (Tokyo). 2000. 48:1286–1292.

11. Li J, Lim SS, Lee ES, Gong JH, Shin D, Kang IJ, Kang YH. Isoangustone A suppresses mesangial fibrosis and inflammation in human renal mesangial cells. Exp Biol Med (Maywood). 2011. 236:435–444.

12. Seon MR, Park SY, Kwon SJ, Lim SS, Choi HJ, Park H, Lim DY, Kim JS, Lee CH, Kim J, Park JH. Hexane/ethanol extract of Glycyrrhiza uralensis and its active compound isoangustone A induce G1 cycle arrest in DU145 human prostate and 4T1 murine mammary cancer cells. J Nutr Biochem. 2012. 23:85–92.

13. Seon MR, Lim SS, Choi HJ, Park SY, Cho HJ, Kim JK, Kim J, Kwon DY, Park JH. Isoangustone A present in hexane/ethanol extract of Glycyrrhiza uralensis induces apoptosis in DU145 human prostate cancer cells via the activation of DR4 and intrinsic apoptosis pathway. Mol Nutr Food Res. 2010. 54:1329–1339.

14. Seo JY, Park J, Kim HJ, Lee IA, Lim JS, Lim SS, Choi SJ, Park JH, Kang HJ, Kim JS. Isoalantolactone from Inula helenium caused Nrf2-mediated induction of detoxifying enzymes. J Med Food. 2009. 12:1038–1045.

15. Lee YS, Kim SH, Kim JK, Shin HK, Kang YH, Park JH, Lim SS. Rapid identification and preparative isolation of antioxidant components in licorice. J Sep Sci. 2010. 33:664–671.

16. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996. 239:70–76.

17. Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull (Tokyo). 1988. 36:2090–2097.

18. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999. 26:1231–1237.

19. Kim HJ, Suh HJ, Kim JH, Park S, Joo YC, Kim JS. Antioxidant activity of glyceollins derived from soybean elicited with Aspergillus sojae. J Agric Food Chem. 2010. 58:11633–11638.

20. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999. 27:612–616.

21. Li WG, Miller FJ Jr, Brown MR, Chatterjee P, Aylsworth GR, Shao J, Spector AA, Oberley LW, Weintraub NL. Enhanced H(2)O(2)-induced cytotoxicity in "epithelioid" smooth muscle cells: implications for neointimal regression. Arterioscler Thromb Vasc Biol. 2000. 20:1473–1479.
22. Rao AR, Sarada R, Baskaran V, Ravishankar GA. Antioxidant activity of Botryococcus braunii extract elucidated in vitro models. J Agric Food Chem. 2006. 54:4593–4599.

23. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978. 52:302–310.
24. Jadhav HR, Bhutani KK. Antioxidant properties of Indian medicinal plants. Phytother Res. 2002. 16:771–773.

25. Shoemaker M, Hamilton B, Dairkee SH, Cohen I, Campbell MJ. In vitro anticancer activity of twelve Chinese medicinal herbs. Phytother Res. 2005. 19:649–651.

26. Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001. 131:955S–962S.

27. Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011. 82:1807–1821.

28. Gibellini L, Pinti M, Nasi M, Montagna JP, De Biasi S, Roat E, Bertoncelli L, Cooper EL, Cossarizza A. Quercetin and cancer chemoprevention. Evid Based Complement Alternat Med. 2011. 2011:591356.

29. Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003. 43:89–143.

30. Davis JM, Murphy EA, Carmichael MD. Effects of the dietary flavonoid quercetin upon performance and health. Curr Sports Med Rep. 2009. 8:206–213.

31. Cuendet M, Guo J, Luo Y, Chen S, Oteham CP, Moon RC, van Breemen RB, Marler LE, Pezzuto JM. Cancer chemopreventive activity and metabolism of isoliquiritigenin, a compound found in licorice. Cancer Prev Res (Phila). 2010. 3:221–232.

32. Fu Y, Hsieh TC, Guo J, Kunicki J, Lee MY, Darzynkiewicz Z, Wu JM. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem Biophys Res Commun. 2004. 322:263–270.

33. Heikens J, Fliers E, Endert E, Ackermans M, van Montfrans G. Liquorice-induced hypertension--a new understanding of an old disease: case report and brief review. Neth J Med. 1995. 47:230–234.

34. Sardi A, Geda C, Nerici L, Bertello P. Rhabdomyolysis and arterial hypertension caused by apparent excess of mineralocorticoids: a case report. Ann Ital Med Int. 2002. 17:126–129.
35. Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008. 22:709–724.

36. Harborne JB. Nature, distribution and function of plant flavonoids. Prog Clin Biol Res. 1986. 213:15–24.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download