Abstract
Figures and Tables
 | Fig. 1
The effect of L. cladonioides on cell viability in 3T3-L1 cells. Differentiated 3T3-L1 cells were incubated with L. cladonioides at the indicated various concentrations for 24, 48, and 72 hr. Data are expressed as percent growth rate of cells cultured in the presence of L. cladonioides, compared with untreated control cells, taken as 100% (mean ± SD). Statistical analysis was performed using a one-way ANOVA with repeated measures followed by Duncan's multiple range tests. Letters with different superscripts are significantly different (P < 0.05) among groups by one-way ANOVA. |
 | Fig. 2
The effect of L. cladonioides on apoptosis/necrosis in 3T3-L1 cells. Differentiated 3T3-L1 cells were incubated with L. cladonioides at the indicated various concentrations for 72 hr by Annexin V/PI double staining. Annexin V FITC-/PI- (Normal cells), Annexin V FITC+/PI- (Early apoptotic cells), Annexin V FITC+/PI+ (Late apoptotic cells), and Annexin V FITC-/PI+ (Necrotic cells). Statistical analysis was performed using one-way ANOVA with repeated measures followed by Duncan's multiple range tests. Letters with different superscripts are significantly different (P < 0.05) among groups by one-way ANOVA. |
 | Fig. 3
Effect of L. cladonioides on lipid accumulation in 3T3-L1 cells. Differentiated 3T3-L1 cells were treated with various concentrations of L. cladonioides. As a positive control, TNF-α (1 ng/mL) was used. After 72 hr, lipid accumulation was evaluated using Oil Red O staining. Each experiment was performed in triplicate. Values are means ± SD. Statistical analysis was performed using one-way ANOVA with repeated measures followed by Duncan's multiple range tests. Letters with different superscripts are significantly different (P < 0.05) among groups by one-way ANOVA. |
 | Fig. 4
The effect of L. cladonioides on glycerol release in 3T3-L1 cells. Differentiated 3T3-L1 cells were treated with various concentrations of L. cladonioides. Cell supernatants were collected after 24, 48, and 72 hr and free glycerol release was assayed. Each experiment was performed in triplicate. Values are mean ± SD. Statistical analysis was performed using a one-way ANOVA with repeated measures followed by Duncan's multiple range tests. Letters with different superscripts are significantly different (P < 0.05) among groups by one-way ANOVA. |
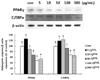 | Fig. 5
The effect of L. cladonioides on the expression of the adipogenic enzyme in 3T3-L1. Differentiated 3T3-L1 cells were treated with various concentrations of L. cladonioides. After 72 hr, cells were collected and they were washed in PBS and lysed in RIPA buffer. Each experiment was performed in triplicate. Values are mean ± SD. Statistical analysis was performed using one-way ANOVA with repeated measures followed by Duncan's multiple range tests. Letters with different superscripts are significantly different (P < 0.05) among groups by one-way ANOVA. |
 | Fig. 6
Comparisons of body weight in DIO mice treated with L. cladonioides. ND, Normal diet; HFD, high fat diet; CA, caffeine; LC, L. cladonioides. Values for the normal diet group were measured, but excluded from statistics. Data were presented as the mean ± SD. Statistical analysis was performed using a one-way ANOVA with repeated measures followed by Duncan's multiple range tests. Letters with different superscripts are significantly different (P < 0.05) among groups by one-way ANOVA. |
Table 1

*Typical analysis of cholesterol in lard = 0.95 mg/gram
1)ND, normal diet (Cholesterol 18 mg/kg diet)
2)HFD, high fat diet (Cholesterol 196.5 mg/kg diet)
3)Mineral mix S10026: Magnesium oxide; Mg 0.5 mg, Magnesium sulfate; S 0.33 mg, Sodium chloride; Na 1.0 mg, Cl 1.6 mg, Chromium KSO4; Cr 2.0 mg, Cupric carbonate; Cu 6.0 mg, Potassium lodate; I 0.2 mg, Ferric citrate; Fe 45.0 mg, Manganous carbonate; Mn 59.0 mg, Sodium selenate; Se 0.18 mg, Zinc carbonate; Zn 29.0 mg, Sodium fluoride; F 0.9 mg, Ammonium molybdate; Mo 1.6 mg
4)Vitamin mix V10001: nicotinic acid; 30 mg, calcium pantothenate; 16 mg, pyridoxine-HCl; 7 mg, thiamin-HCl; 6 mg, riboflavin; 6 mg, folic acid; 6 mg, biotin; 0.2 mg, vitamin E acetate; 50 IU, vitamin B12; 10 µg, vitamin A palmitate; 4,000 IU, vitamin D3; 1,000 IU, menadione Na bisulfate; 0.5 mg
Table 2

ND, Normal diet; HFD, high fat diet; CA, caffeine; LC, L. cladonioides
1)FER, food efficiency ratio = [body weight gain(g)/total food intake(g) × 102]
The values for the normal diet group were measured, but excluded from statistics. Data were presented as the mean ± SD. Statistical analysis was performed using one-way ANOVA followed by Duncan's multiple range tests. Letters with different superscripts in the same row are significantly different (P < 0.05) among groups by one-way ANOVA.
Table 3

ND, Normal diet; HFD, high fat diet; CA, caffeine; LC, L. cladonioides
1)EWAT, epididymal white adipose tissue
2)RWAT, retroperitoneal white adipose tissue.
The values from the normal diet group were measured, but excluded from statistics. Data were presented as the mean ± SD. Statistical analysis was performed using one-way ANOVA followed by Duncan's multiple range tests. Letters with different superscripts in the same row are significantly different (P < 0.05) among groups by one-way ANOVA.
Table 4

ND, Normal diet; HFD, high fat diet; CA, caffeine; LC, L. cladonioides
Values for the normal diet group were measured, but excluded from statistics. Data were presented as the mean ± SD. Statistical analysis was performed using oneway ANOVA followed by Duncan's multiple range tests. Letters with different superscripts in the same row are significantly different (P < 0.05) among groups by one-way ANOVA.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download