Abstract
The hypolipidemic and hypoglycemic effects of two dietary dosages (0.1% and 0.5%) of water and 80% ethanol extracts from hot-air dried Orostachys japonicus A. Berger were evaluated in the serum and organ tissues of streptozotocin-induced diabetic rats. The STZ-induced diabetic groups supplemented with the O. japonicus extracts showed significantly higher body weight compared to a diabetic control group at the end of experiment. The extracts exhibited substantial hypoglycemic effects by significant reductions of fasting blood glucose levels at all time points tested compared to the initial stage before treatment of the extracts. Declines of serum and hepatic triglyceride levels were greater than declines of total cholesterol in the groups treated with the 0.5% O. japonicus extract (DBW2 and DBE2) when compared to the DBC group. Hepatic glycogen content was higher in the groups treated with O. japonicus extract, while lipid peroxide content was decreased in these treated groups compared to the DBC group. Hepatic antioxidant activity was significantly increased in the groups supplemented with the O. japonicus ethanol extract. The hypolipidemic and hypoglycemic effects of the O. japonicus ethanol extract were significantly greater than the effects of the water extract. Based on this study, it seems that O. japonicus ethanol extract, due to its higher phenolic and flavonoid components than the water extract, may control blood glucose and alleviate hyperlipidemia in diabetes.
Diabetes mellitus is a chronic and progressive disorder characterized by hyperglycemia and metabolic disturbances of carbohydrate, protein, and lipids [1]. In particular, type 2 diabetes has increased over recent years in Korea, in accordance with age, obesity, and smoking [2]. This is related to hepatic insulin resistance, which leads to dyslipidemia and cardiovascular disease, such as raised triglyceride and low density lipoprotein (LDL) levels, and a reduced high density lipoprotein (HDL) level in serum [3]. Cardiovascular disease as a severe chronic complication is 2-4 times higher in diabetic patients than the healthy population [4]. Because modern medicine has side-effects and no means by which to completely cure diabetes mellitus [5], elderly people in Korea have used traditional herbs as alternative therapeutic medicines to prevent cardiovascular disease complications in diabetes. The potential anti-hyperlipidemic [6,7] and antidiabetic effects [8,9] of several traditional plants have been reported in Korea.
Orostachys japonicus (O. japonicus) A. Berger (Crassulaceae), commonly called "wa-song" in Korea, is a herbal folk remedy for treatment or prevention of chronic diseases including hepatitis, hemorrhoids, hematemesis, and cancer [10]. Phytochemicals such as triterpense [11,12], phytosterols [12], and flavonoids [13-15] have been isolated from this plant. We determined the antioxidant activities of O. japonicus extract by different drying conditions such as sun-drying, hot-air drying, and freeze drying, and found that the hot-air dried O. japonicus had the highest antioxidant activity due to a higher content of phenolic compounds [16]. It was also found to delay antioxidant activities for lipid peroxidation reactions in a lipid emulsion model system [17]. We recently reported that quercetin and kaempferol derivatives are major flavonoid components of O. japonicus, and these components may be important contributors to free radical scavenging, in vitro [15]. In particular, quercetin also suppresses glucose levels in diabetic rats, and reduces plasma cholesterol and triglycerides probably by enhancing insulin release from pancreatic islets in diabetic rats [18,19].
O. japonicus A. Berger was obtained from local medicinal herb cultivators in Jinju city, Korea, during July-August, 2006. It was authenticated by an herbal doctor from an oriental medicine clinic located in Jinju city as well as professor Jin Ho Kang, Research Institute of Life Science in Gyeongsang National University. The whole tissue of the fresh plant was rinsed with tap water, dried by a hot-air dryer (CF-21WF, JEIOTECH, Daejeon, Korea) at 70℃ for 4-5 h, powdered by 80-100 mesh, and stored at -40℃.
The powder of hot-air dried O. japonicus (100 g) was extracted in water and 80% ethanol (1 L) at 70℃ for 3 h, respectively. The extracts were filtered through filter paper (Whatman No. 6, Whatman International Ltd., Maidstone, UK) after centrifuging (Mega 17R, HANILE, Inchon, Korea) at 3,000 rpm for 10 min. The residues were extracted two times. The supernatants were concentrated under reduced pressure using a rotary evaporator, and lyophilized to give a powder (yields were 2.76% and 0.72% in the water and 80% ethanol extract of O. japonicus, respectively). They were stored at -40℃ and used for animal treatments.
Male albino Sprague-Dawley (SD) rats were purchased from Samtako Bio Korea Inc. (Osan, Korea). Anti-diabetic studies were performed on healthy 5 week-old rats that weighed 150 ± 10 g. All rats were kept in automatic chambers (DJ1-252-2, Daejong Instrument Industry Co. Ltd., Seoul, Korea) with a room temperature of 22 ± 2℃, humidity of 50 ± 5%, and a constant light and dark cycle at 12 h (07:00-19:00) each. To help them adapt to the laboratory conditions, the rats were fed a standard pellet diet (Samtako Bio Korea Inc. Osan, Korea) with water ad libitum the first week prior to the experiment.
We used STZ (Sigma Co., St. Louis, MO, USA) as a diabetic model to maintain a mild and stable form of diabetes during the experimental period; this induced diabetic syndrome is partly similar to type 2 diabetes in humans [21]. The STZ was freshly dissolved in citrate buffer (0.01 M, pH 4.5) and immediately injected intraperitoneally with a single dose of 50 mg/kg in overnight fasted SD rats. After 48 h of STZ injection, diabetes was confirmed by blood samples collected from the tip of the tail using a blood glucometer (ACCU-CHEK, Mannheim, Germany). The rats with a fasting blood glucose level above 300 mg/dL were considered diabetic and were used in the experiment.
The normal group (NC) served as nondiabetic control rats treated with saline instead of STZ and a normal diet. The diabetic rats were randomly divided into 5 groups of 7 rats each. Of the diabetic groups, DBC as the diabetic control group received only a normal diet. The DBW1 and DBW2 groups received diets supplemented with 0.1% and 0.5% of O. japonicus water extract, respectively. The DBE1 and DBE2 groups received diets supplemented with 0.1% and 0.5% of O. japonicus ethanol extract, respectively. The extracts were mixed with a normal diet (rodent AIN-76 basal diet), and all animals had free access to eat. The composition of the diet is shown in Table 1.
Food and water intake were monitored daily at the same time during the experimental period. The food efficiency ratio (FER) was calculated as the total body weight gain (g)/total food intake amount (g) during the experimental period. At the end of the experimental period, the rats were sacrificed following an overnight fast by an overdose of ethyl ether. Blood samples were collected from the heart and the serum was collected by centrifugation at 5,000 rpm for 10 min at 4℃. The serum was stored at -70℃ prior to analysis. The liver, kidney, and heart were excised rapidly, and chilled and rinsed in ice cold 0.9% aqueous sodium chloride. They were stored at -70℃ prior to analysis.
Changes in fasting blood glucose (FBG) were measured using a glucometer with serum obtained from the tail vein every week. Serum total cholesterol (TC), triglyceride (TG), and high density lipoprotein cholesterol (HDL-C) levels were determined by a blood biochemical analyzer (DRI-CHEM 3500i/3500s automated clinical chemical analyzer, Fuji, Tokyo, Japan) using appropriate kit reagents (TCHO-PIII, TG-PIII, and HDL-C-PIII, Fuji, Tokyo, Japan), respectively. Low density lipoprotein cholesterol (LDL-C) levels were calculated by Friedewald's equation: TC-(HDL-C + TG/5) [22]. The atherogenic index (AI) was calculated by the following equation: (TC-HDL-C)/HDL-C [23]. Cardiovascular risk factors (CRF) were calculated by the ratio of HDL-C to TC (TC/HDL-C) [24]. GOT (glutamic oxaloacetic transaminase) and GPT (glutamic pyruvic transaminase) activities in serum were measured using GOT-PIII and GPT-PIII kit reagents (Fuji), respectively.
Lipid levels of the organ tissues (liver, kidney, and heart) were determined according to the method of Folch et al. [25]. Each tissue (0.5 g) sample was homogenized with 30 mL of chloroform: methanol (2:1, v/v) solution, extracted for 24 h, and filtrated with filter paper (Whatman No. 6, Whatman International Ltd.). The filtrate was completely dried by a rotary evaporator at 30-40℃, and the residue was used to determine TC and TG.
Protein levels in the rats were determined by the method of Lowry et al. [26]. The glycogen contents of the rat livers were determined using anthrone reagent (Fluka Chemical Co., Buchs, Switzerland) [27]. D-glucose (Sigma Co.) was used as a standard for the calibration curve and glycogen content was expressed as mg/g of wet tissue. Lipid peroxide levels were determined according to the method of Mihara and Uchiyama [28]. 1,1,3,3-tetraethoxypropan (Sigma Co.) was used as a standard.
To analyze hepatic antioxidant activity, liver tissue (1 g) was homogenized in 10 mL of cold 1.5% KCl solution to obtain a 10% (w/v) homogenate. The antioxidant activity was assayed according to the method of Lim et al. [29] with slight modifications. Each sample (200 µL) was thoroughly mixed with 1 mL of Tris-HCl buffer (100 mM, pH 7.4), and 2 mL of 1,1-diphenyl-2-picrylhydrazyl (DPPH, Sigma Co., 2.5 mg/100 mL ethanol) was added rapidly. The mixture was incubated at 37℃ for 15 min before it was added to 4 mL of chloroform. After centrifugation at 5,000 rpm for 5 min at 4℃, the absorbance of the chloroform layer was measured at 517 nm and chloroform was used as a blank. The antioxidant activity was expressed as the scavenging ratio of DPPH reagent color (%).
No significant changes were observed in baseline body weight values. There were significant differences in final body weight as well as food and water intake in the DBC group compared to the NC group (Table 2). The fed amounts of O. japonicus extract from daily food intake were calculated as 24.2 mg/rat/day in the DBW1 and DBE1 groups, 115.5 mg/rat/day in the DBW2 group, and 103.5 mg/rat/day in the DBE2 group. The STZ-induced diabetic groups supplemented with the O. japonicus extracts had significantly increased body weight compared to the DBC group, although total food intake was not significantly different. The FERs of groups treated with the O. japonicus ethanol extract were better than those treated with the water extract.
The water and ethanol extracts of O. japonicus were administered at different doses (0.1% and 0.5%, respectively) in STZ-induced diabetic rats for 4 weeks. The effects of the extracts on weekly FBG levels are presented in Fig. 1. The FBG level of the NC group during the 4 weeks was about 95.5 mg/dL, while FBG in the STZ-induced diabetic groups was about 328.8 mg/dL at the initial stage before extract administration. The supplementation of O. japonicus extract in the STZ-induced diabetic rats produced significant suppression of FBG levels at all points tested compared to the initial stage. Moreover, at the end of the 4 weeks of treatment, better declines in FBG level were observed in the DBW2, DBE1, and DBE2 groups compared to the DBW1 group (P < 0.05).
The levels of serum lipids such as TC, TG, HDL-C, and LDL-C, as well as AI and CRF values at the end of the experimental period are presented in Table 3. Significant differences in TC, TG, HDL-C, and LDL-C were observed between the DBC group and groups treated with O. japonicus extract. However, TC levels were not significantly different according to the dosage amounts of the extract. The TG levels of the DBW2 and DBE2 groups declined by 15.8% and 28.6% compared to the DBC group, respectively. TG levels in the DBE2 group were recovered to normal values. HDL-C levels were significantly decreased in the DBC group with respect to the NC group, while there was a significant elevation of HDL-C in all groups treated with the O. japonicus extracts. This was especially apparent in the DBE2 group (P < 0.05). AI and CRF values were significantly decreased in the groups treated with O. japonicus extract compared to the DBC group.
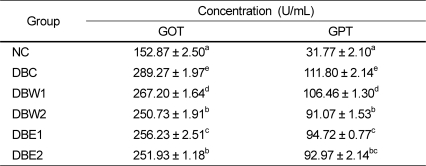
Serum GOT and GPT activities are shown in Table 4. All groups treated with the O. japonicus extract had significant decreases in GOT and GPT activities compared to the DBC group. And these decreased activities occurred in a dose-dependent manner. Furthermore, with 0.1% supplementation of the O. japonicus extract, the group fed the ethanol extract (DBE1) had more significant effects than the group fed the water extract (DBW1).
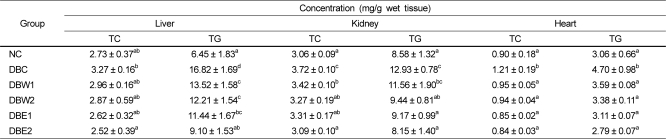
The lipid profiles of organ tissues are shown in Table 5. Lipid levels in the tissues were significantly decreased in the groups supplemented with O. japonicus extract compared to the DBC group. TG levels in the liver tissue were reduced about 27.4% and 45.9% in the DBW2 (12.21 mg/g) and DBE2 (9.10 mg/g) groups as compared to the DBC group (16.82 mg/g), respectively. In the DBE2 group, TG levels in the kidney and heart tissue were decreased to 37.0% and 40.6%, respectively, compared to the DBC group.
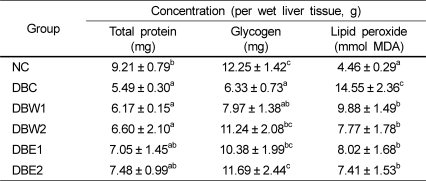
As presented in Table 6, the hepatic protein contents of the groups supplemented with O. japonicus extract were not significantly different from that of the DBC group. The hepatic glycogen contents of the DBW2, DBE1, and DBE2 groups were significantly higher than that of the DBC group. In particular, glycogen content in the DBE2 group was similar to that in the NC group. Lipid peroxide content was significantly increased in the DBC group with respect to the NC group, but significantly lower in the groups treated with O. japonicus extract compared to the DBC group. However, there were no significant differences among the groups fed the O. japonicus extracts.
Hepatic antioxidant activity was tested by the DPPH radical scavenging ratio and is shown in Fig. 2. Hepatic antioxidant activity was significantly increased in the groups treated with the O. japonicus extracts compared to the DBC group. The ethanol extract of O. japonicus was more effective at elevating antioxidant activity than the water extract.
Several reviews on the bioactivity of flavonoids from O. japonicus and their potential therapeutic uses for diabetic control have been published [30-32]. Total flavonoid content (361.6 mg/100 g) from O. japonicus, including quercetin derivatives (50.80-694.03 mg/kg) and kaempferol derivatives (402.09-408.19 mg/kg), has been determined by HPLC-MS/MS. Quercetin and kaempferol derivatives occupy about 53% of the total flavonoid content in O. japonicus [15]. Moreover, polyphenol and flavonoid components in the plant were found to be closely connected to its antioxidant activities [15-17,20]. However, the relationship of dietary flavonoids to type 2 diabetes therapy is less well-studied in Korea. Therefore, in this study, we surmised that these compounds would be able to cause hypolipidemic and hypoglycemic effects in STZ-induced diabetic rats.
Table 2 reveals the phenomena of polyphagia (increased appetite) and polydipsia (increased thirst and consequent elevated water intake) by the supplementation of O. japonicus extracts, and body weight loss was decreased significantly (P < 0.05). Body weights in the groups treated with the O. japonicus ethanol extract (DBE1 and DBE2 groups) increased about 8-9%, while in the DBC group body weight was reduced by about 13.2% at the end of experimental period compared to initial body weight. The ethanol extract was more effective than the water extract in alleviating diabetic symptoms, similar to the results reported by Kang et al. [33]. FBG levels in the DBC group during the 4 weeks were in the range of 325.4-331.3 mg/dL (Fig. 1). However, the FBG levels of the diabetic rats were significantly decreased by supplementation of O. japonicus extract compared to the DBC group, suggesting that the mode of anti-diabetic activity by flavonoids is possibly related to hypoglycemic action. Flavonoids, with a C-3 position, have inhibitory action against aldose reductase [34], which can be considered hypoglycemic action [35]. This activity may be increased based on their ability to establish an H bound between the compound and its target. It was also reported that the basic flavone structure of flavonoids possesses anti-diabetic activity [30].
The STZ-induced diabetic rats exhibited obvious abnormalities of lipid metabolism as evidenced from significant elevations of serum TC, TG, and LDL-C as well as AI and CRF values, and reduced HDL-C levels. Supplementation of the O. japonicus extracts for 4 weeks to the diabetic rats caused hypolipidemic action through significant depression of TG levels in the serum and organ tissues of the groups treated with the ethanol extract compared to the water extract, suggesting that the ethanol extract would be effective against cardiovascular disease and/or atherosclerosis, and perhaps control dyslipidemia in diabetes. These results are similar to previous reports depicting flavonoids as potential hypolipidemic agents, because the lowering effects of flavonoids on TG and LDL-C levels were greater than the effect on TC level in diabetic rats [3,30,36]. Therefore, the reduction of serum TG following treatment with O. japonicus might also facilitate glucose oxidation and utilization, and subsequently lead to the reduction of hyperglycemia. The increased gluconeogenesis and ketogenesis in diabetes might be due to elevated transaminase activity such as by GOT and GPT [37]. Abnormal serum GOT and GPT activities indicate tissue damage by toxicants or disease and are of clinical and toxicological importance [38]. Treatment with O. japonicus for 4 weeks reduced these enzyme activities compared to the non-treated DBC group. We expect that continuous supplementation of O. japonicus extracts may result in recovery of impaired hepatic function.
In the DBW2 and DBE2 groups treated with the water and ethanol extracts at 0.5%, the decline in hepatic TG was better than that of TC in the DBC group (Table 5). The ethyl acetate fraction from O. japonicus decreased serum and hepatic TG levels about 45.7% and 32.4%, respectively, in comparison to the diabetic control group. These results suggest that this fraction from O. japonicus contains certain sterols and flavonoids such as kaempferol, astragalin, and isoquercitrin, and administration of this fraction is associated with reduced plasma clotting times [36].
A decline status of hepatic glycogen content has frequently been observed in diabetes [39]. Higher glucose levels and lower hepatic glycogen content could be attributed to less availability of the active form of glycogen synthetase [36]. Especially, since STZ causes selective destruction of β-cells of the pancreas, it could be predicted that glycogen levels in liver and muscle tissues decrease as glucose influx into the liver is inhibited in the absence of insulin. Our study shows that glycogen levels in the groups treated with the O. japonicus ethanol extract were recovered to the levels of normal rats (Table 6). This indicates that one of the bioactivities of O. japonicus might be the management of hepatic glycogenesis, and quercetin could potentially have hypoglycemic activity [19].
Many studies have reported significantly increased lipid peroxide contents in the liver and kidney tissues of diabetic rats [40,41]. In STZ-induced diabetic animals, powerful free radical oxidants by STZ result in increased serum lipid peroxide content due to the oxidation of cells [42]. The lower content of lipid peroxide by supplementation of O. japonicus extract may be closely connected to its polyphenol and flavonoid content [17], expecting that these components decrease lipid peroxide content in tissues [43]. The hepatic antioxidant activity of the ethanol extract of O. japonicus was more efficient than that of the water extract.
Overall, we found that O. japonicus extract has multiple blood glucose lowering and hypolipidemic effects, and had hypothesized that it would increase antioxidative system activity in vivo the as same as it does in vitro. Further systematical anti-diabetic studies should be performed to elucidate the exact mechanism(s) and to develop O. japonicus as a potential material for anti-diabetic activity.
References
1. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003; 26:S5–S20. PMID: 12502614.
2. Chung HR, Pérez-Escamilla R. Risk factors of type 2 diabetes among Korean adults: The 2001 Korean national health and nutrition examination survey. Nutr Res Pract. 2009; 3:286–294. PMID: 20098581.

3. Carmena R. Type 2 diabetes, dyslipidemia, and vascular risk: Rationale and evidence for correcting the lipid imbalance. Am Heart J. 2005; 150:859–870. PMID: 16290951.

4. Ko M, Kim MT, Nam JJ. Assessing risk factors of coronary heart disease and its risk prediction among Korean adults: The 2001 Korea national health and nutrition examination survey. Int J Cardiol. 2006; 110:184–190. PMID: 16412525.

5. Park SY, Han JS. Effects of web-based nutrition counseling on nutrient intake and blood glucose in Type II diabetic patients. J Korean Soc Food Sci Nutr. 2005; 34:1398–1406.
6. Yoon JA, Son YS. Effects of Opuntia ficus-indica complexes B (OCB) on blood glucose and lipid metabolism in streptozotocin-induced diabetic rats. Korean J Food Nutr. 2009; 22:48–56.
7. Han HK, Yoon SJ, Kim GH. Effects of compositae plants on plasma glucose and lipid level in streptozotocin induced diabetic rats. J Korean Soc Food Sci Nutr. 2009; 38:674–682.

8. Kim JY, Park JY, Lee KU. Diabetes and traditional medicine: effect of several traditional drugs on the plasma glucose levels in streptozotocin-induced diabetic rats. J Korean Diabetes Assoc. 1994; 18:377–381.
9. Park MJ, Han JS. Antioxidant activity of medicinal plant extracts used as folk remedies by diabetic patients. J Food Sci Nutr. 2004; 9:167–173.

10. Kim JK. Illustrated Natural Drugs. 1984. Seoul: Namsandang Publishing Co.;p. 447.
11. Park HJ, Young HS, Kim JO, Rhee SH, Choi JS. A study on the chemical constituents of Orostachys japonicus A. Berger. Korean J Pharmacogn. 1991; 22:78–84.
12. Park HJ, Lim SC, Lee MS, Young HS. Triterpene and steroids from Orostachys japonicus. Korean J Pharmacogn. 1994; 25:20–23.
13. Park HJ, Moon SH, Park KY, Choi JS, Chung HY, Young HS, Suh SS. Antimutagenic effect of Orostachys japonicus. Yakhak Hoeji. 1991; 35:253–257.
14. Park HJ, Young HS, Park KY, Rhee SH, Chung HY, Choi JS. Flavonoids from the whole plants of Orostachys japonicus. Arch Pharm Res. 1991; 14:167–171.
15. Lee JH, Lee SJ, Park S, Kim HK, Jeong WY, Choi JY, Sung NJ, Lee WS, Lim CS, Kim GS, Shin SC. Characterisation of flavonoids in Orostachys japonicus A. Berger using HPLC-MS/MS: Contribution to the overall antioxidant effect. Food Chem. 2011; 124:1627–1633.
16. Lee SJ, Seo JK, Shin JH, Lee HJ, Sung NJ. Antioxidant activity of wa-song (Orostachys japonicus A. Berger) according to drying methods. J Korean Soc Food Sci Nutr. 2008; 37:605–611.
17. Lee SJ, Cha JY, Shin JH, Chung MJ, Sung NJ. Antioxidant effect of wa-song (Orostachys japonicus A. Berger) extracts on edible oil and fat. J Life Sci. 2008; 18:1106–1114.
18. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003; 135C:357–364. PMID: 12927910.

19. Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011; 5:107–111. PMID: 21556223.

20. Lee SJ, Song EJ, Lee SY, Kim KBWR, Kim SJ, Yoon SY, Lee CJ, Ahn DH. Antioxidant activity of leaf, stem and root extracts from Orostachys japonicus and their heat and pH stabilities. J Korean Soc Food Sci Nutr. 2009; 38:1571–1579.
22. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502. PMID: 4337382.

23. Haglund O, Loustarinen R, Wallin R, Wibell I, Saldeen T. The effect of oil on triglycerides, cholesterol, fibrinogen and malondialdehyde in mand supplemented with vitamin. Eur J Nutr. 1991; 121:165–172.
24. Kang SM, Shim JY, Hwang SJ, Hong S, Jang HE, Park MH. Effects of Saengshik supplementation on health improvement in diet-induced hypercholesterolemic rats. J Korean Soc Food Sci Nutr. 2003; 32:906–912.
25. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509. PMID: 13428781.

26. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275. PMID: 14907713.

27. Carroll NV, Longley RW, Roe JH. The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem. 1956; 220:583–593. PMID: 13331917.

28. Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978; 86:271–278. PMID: 655387.
29. Lim BO, Seo TW, Shin HM, Park DK, Kim SU, Cho KH, Kim HC. Effect of betulae platyphyllae cortex on free radical in diabetic rats induced by streptozotocin. Korean J Herbol. 2000; 15:69–77.
30. Torres-Piedra M, Ortiz-Andrade R, Villalobos-Molina R, Singh N, Medina-Franco JL, Webster SP, Binnie M, Navarrete-Vázquez G, Estrada-Soto S. A comparative study of flavonoid analogues on streptozotocin-nicotinamide induced diabetic rats: Quercetin as a potential antidiabetic agent acting via 11beta-hydroxysteroid dehydrogenase type 1 inhibition. Eur J Med Chem. 2010; 45:2606–2612. PMID: 20346546.
31. Sharma B, Balomajumder C, Roy P. Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food Chem Toxicol. 2008; 46:2376–2383. PMID: 18474411.
32. Lü H, Chen J, Li WL, Ren BR, Wu JL, Zhang HQ. Hypoglycemic effect of the total flavonoid fraction from Folium Eriobotryae. Phytomedicine. 2009; 16:967–971. PMID: 19427773.
33. Kang SJ, Bao CL, Park S, Kim AJ. Hypoglycemic effect of Rehmannie radix preparata (Sookjihwang) extract in streptozotocin-induced diabetic rats. Nutr Res Pract. 2010; 4:438–442. PMID: 21103092.
34. Singab AN, El-Beshbishy HA, Yonekawa M, Nomura T, Fukai T. Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005; 14:333–338. PMID: 15885940.
35. Chen F, Nakashima N, Kimura I, Kimura M. Hypoglycemic activity and mechanisms of extracts from mulberry leaves (Folium mori) and Cortex mori Radicis in streptozotocin-induced diabetic mice. Yakugaku Zasshi. 1995; 115:476–482. PMID: 7666358.
36. Kim SG, Choi J, Park HJ, Lee SM, Jung HJ. Anti-hyperlipidemic effects of the flavonoid-rich fraction from the methanol extract of Orostachys japonicus in rats. Korean J Pharmacogn. 2009; 40:51–58.
37. Felig P, Marliss E, Ohman JL, Cahill CF Jr. Plasma amino acid levels in diabetic ketoacidosis. Diabetes. 1970; 19:727–728. PMID: 4990780.

38. Singh SN, Vats P, Suri S, Shyam R, Kumria MM, Ranganathan S, Sridharan K. Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J Ethnopharmacol. 2001; 76:269–277. PMID: 11448549.
39. Grover JK, Vats V, Yadav S. Effect of feeding aqueous extract of Pterocarpus marsupium on glycogen content of tissues and the key enzymes of carbohydrate metabolism. Mol Cell Biochem. 2002; 241:53–59. PMID: 12482025.
40. Latha M, Pari L. Preventive effects of Cassia auriculata L. flowers on brain lipid peroxidation in rats treated with streptozotocin. Mol Cell Biochem. 2003; 243:23–28. PMID: 12619885.
41. Venkateswaran S, Pari L. Antioxidant effect of Phaseolus vulgaris in streptozotocin-induced diabetic rats. Asia Pac J Clin Nutr. 2002; 11:206–209. PMID: 12230234.
42. Wakame K. Protective effects of active hexose correlated compound (AHCC) on the onset of diabetes induced by streptozotocin in the rat. Biomed Res. 1999; 20:145–152.

43. Jang MJ, Rhee SJ. Antioxidant effects of mixture of mulberry leaves and silkworm powder on the plasma and liver in streptozotocin-induced diabetic rats. J Food Sci Nutr. 2004; 9:346–351.
Fig. 1
Changes in fasting blood glucose levels in STZ-induced diabetic rats supplemented with O. japonicus extracts during 4 weeks. Different superscripts indicate a significant difference (P < 0.05). Significant differences for the DBW2 and DBE1 groups show the same letters.
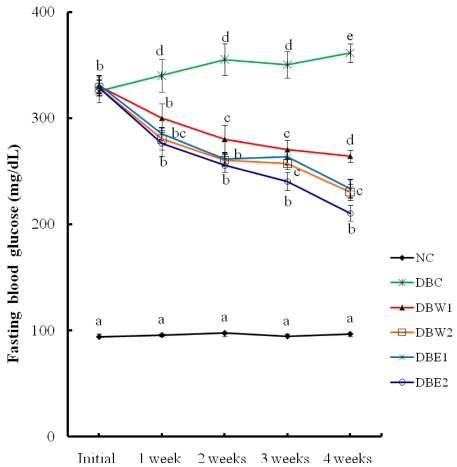
Fig. 2
Hepatic antioxidant activity in STZ-induced diabetic rats supplemented with O. japonicus extracts for 4 weeks. Different superscripts indicate a significant difference (P < 0.05) among the groups.
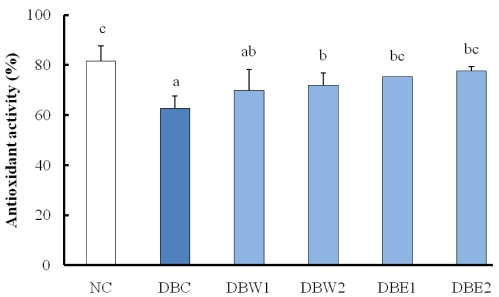
Table 1
Composition of diets (%)
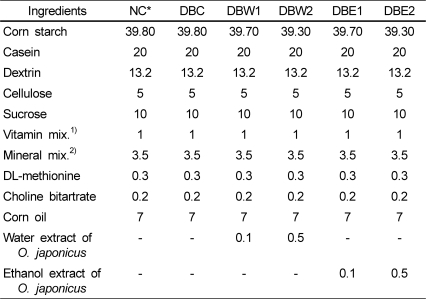
1,2)Vitamin mixture and mineral mixture were prepared according to AIN-76™ NC, no induced diabetes; DBC, STZ-induced diabetes; DBW1 and DBW2, groups with diabetes that received 0.1% and 0.5% water extracts of O. japonicus, respectively; DBE1 and DBE2, groups with diabetes that received 0.1% and 0.5% ethanol extracts of O. japonicus, respectively
Table 2
Body weight, food and water intake, and FER in STZ-induced diabetic rats supplemented with O. japonicus extracts for 4 weeks
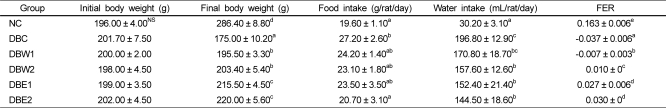
Table 3
Serum lipid profiles of STZ-induced diabetic rats supplemented with O. japonicus extracts for 4 weeks
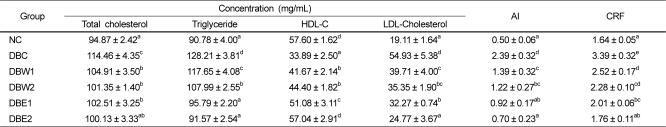
Table 4
GOT and GPT activities in serum of STZ-induced diabetic rats supplemented with O. japonicus extracts for 4 weeks




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download