Abstract
This study was performed to investigate the effect of desalinated underground seawater (named as 'magma seawater', MSW) of Jeju Island in Korea on lipid metabolism and antioxidant activity. MSW was collected from underground of Han-Dong in Jeju Island, and freely given to high fat diet (HFD)-fed C57BL/6 mice for 10 weeks. Although there were no significant differences in the body weight changes and plasma lipid levels, hepatic triglyceride levels were significantly lower in the MSW group than in the normal tap water (TW)-drunken control group. Furthermore, the activity of fatty acid synthase (FAS) was significantly decreased and carnitine palmitoyltransferase (CPT) activity was increased in MSW group compared to TW group. Similarly, real-time PCR analysis revealed that mRNA expressions of lipogenic genes were lowered in MSW groups compared to the control group. In a morphometric observation on the liver tissue, accumulation of fats was remarkably reduced in MSW group. Meanwhile, in vitro assay, free radical scavenging activity measured by using diphenylpicrylhydrazyl (DPPH) was increased in MSW group. The 2'-7'-dichlorofluorescein diacetate (DCF-DA) staining followed with fluorescent microscopy showed a low intensity of fluorescence in MSW-treated HepG2 cells, compared to TW-treated HepG2 cells, which indicated that the production of reactive oxygen species by tert-butyl hydroperoxide (t-BHP) in HepG2 cells was decreased by MSW treatment. The antioxidant effect of MSW on t-BHP-induced oxidative stress in HepG2 cells was supported by the increased activities of intracellular antioxidant enzymes such as catalase and glutathione reductase. From these results, we speculate that MSW has an inhibitory effect on lipogenesis in liver and might play a protective role against cell damage by t-BHP-induced oxidative stress.
Deep seawater has been known to be very pure and rich in minerals such as, magnesium (Mg), calcium (Ca), potassium (K), zinc (Zn), vanadium (V), etc. compared to surface seawater [1,2], which has attracted the attention of deep seawater utilization and application. In relation to health care using deep seawater, the administration of desalinated deep seawater appeared to have beneficial effects for hyperlipidemia, atherosclerosis and cardiovascular hemodynamics of animal model studies [3-7]. Previous study reported that an artificial mineral water containing only Ca and Mg showed higher inhibitory effects on adipocyte differentiation [8]. Furthermore, several studies have showed that consumption of these two minerals may have a protective effect against obesity, atherosclerosis and diabetes mellitus [9-11]. Underground seawater is a kind of underground water but its property and ingredients are different from general underground water because it is originated from seawater. Jeju Island, located at southern part of Korea, has known to have huge pool of underground seawater in its northeast region. Jeju Island had formed by several volcanic explosions and activities between about 1.2 million to 25 thousand years ago, and the underground of northeast region is composed of mainly basaltic rocks with pores favorable to intrusion of seawater. The continuous and repeated intrusion of seawater into the underground of northeast region for a long time has rendered to the current huge pool of underground seawater in Jeju Island.
Epidemiological and experimental evidences have suggested that high dietary fat intake in mice is associated with many physically degenerative diseases. High fat diet can be associated with increased oxidative stress in mammals and subsequently contribute to the high risk of some diseases such as obesity, diabetes and atherosclerosis [12-14]. Therefore, inhibiting oxidative stress can be helpful for preventing the development of these diseases.
Recently we have pumped up the underground seawater of Jeju Island to investigate the application possibilities in various fields. In our knowledge, it is worthy to evaluate the effects of underground seawater due to its high contents of minerals, Ca and Mg. The present study was performed to examine lipid metabolism and antioxidant effects of desalinated underground seawater of Jeju Island in high-fat diet fed C57BL/6 mice and tert-butyl hydroperoxide (t-BHP; oxidative stressor) treated-HepG2 cells.
Underground seawater was pumped up from a depth of 127 m off in Han-Dong area of Jeju Island (Korea), and desalinated by electrodialysis under the condition of 12 mS/cm conductivity. The desalinated underground seawater was named as 'magma seawater (MSW)' and used for the present study, and tap water was used for the control experiment. Major mineral components of MSW and TW are shown in Table 1.
Eight-week-old male C57BL/6 mice from the Korea Research Institute of Bioscience and Biotechnology (KRIBB) in Daejeon, Korea, were used. The mice were housed in plastic cages in a temperature-controlled (22 ± 1℃) room and maintained on a reverse 12h light/dark cycle. The mice were randomly divided into two groups: HFD plus TW as control group and HFD plus MSW were daily administered by free drinking for 10 weeks. The HFD (Dyets No. 100244, Dyets Inc., USA) was based on a modified Western diet and contained 21% (w/w) lard and 0.15% (w/w) cholesterol. The body weight gain was measured once a week. All animal experiments were approved by the Institutional Animal Care and Use Committee and performed in accordance with the institutional guidelines at the KRIBB.
At the end of the experimental period (10 weeks), the mice were sacrificed by cervical dislocation, and blood samples were taken from the orbital venous congestion to determine plasma lipids. The plasma was prepared by centrifugation of blood at 10,000 g for 5 min at 4℃ and stored at -70℃ until analysis. Immediately after mice were killed, the liver was removed and weighed. The tissues were frozen in liquid nitrogen and stored at -70℃ until used for enzyme-based assay. Plasma lipids were measured using a Hitachi 7150 Automatic Serum analyzer (Hitachi Ltd., Japan).
The hepatic lipids were extracted using the procedure developed by Bligh and Dyer [15]. Namely, frozen liver tissue was homogenized in 0.9% NaCl solution and the homogenate was added to CM solution (chloroform : methanol = 1 : 2, v/v). Then, the homogenate was vortexed and centrifuged at 3,000 g for 20 min. The upper phase was aspirated and filtered through a filter paper (Whatman No. 1) to remove any cell debris. The filtered chloroform phase was used for the analysis. The hepatic cholesterol and triglyceride concentrations were analyzed with the enzymatic kit (Asan, Korea) at 500 and 540 nm, respectively.
The hepatic enzyme source was prepared according to the method developed by Hulcher and Oleson [16] with a slight modification. A homogenate was prepared in a buffer containing 0.1 mol/L of triethanolamine, 0.02 mol/L of EDTA, and 2 mmol/L of dithiothreitol, pH 7.0, then centrifuged at 600 g for 10 min to discard any cell debris, and the supernatant was centrifuged at 10,000 g for 20 min at 4℃ to remove the mitochondrial pellet. Thereafter, the supernatant was ultracentrifuged twice at 100,000 g for 60 min at 4℃ to obtain the cytosolic supernatant. The mitochondrial pellet was then redissolved in 800 µl of homogenization buffer and the protein content was determined by the method of Bradford using bovine serum albumin as the standard.
The fatty acid synthase (FAS) activity was measured according to the method of Nepokroeff et al. [17] by monitoring the malonyl-CoA-dependent oxidation of NADPH. A 100 µl of the cytosolic fraction was mixed with 125 mmol/l potassium phosphate buffer (pH 7.0), 165 µmol/l acetyl-CoA, 50 µmol/l malonyl-CoA, 50 µmol/l NADPH, 1 mmol/l β-mercaptoethanol and 1 mmol/l EDTA, which was then measured for 2 min at 340 nm on a spectrophotometer. The activity was represented by the oxidized NADPH nmol/min/mg protein. The carnitinepalmitoyl transferase (CPT) was assayed by following the release of CoA-SH from palmitoyl-CoA using the general thiol reagent (5, 5'-dithiobis 2-nitrobenzoate, DTNB) as described by Markwell et al. [18] with a slight modification. The reaction mixture contained 232 mmol/l Tris-HCl (pH 8.0), 1.1 mmol/l EDTA, 220 µmol/l carnitine, 24 µmol/l DTNB, 7 µmol/l palmitoyl-CoA and 0.09% Triton X-100. The reaction was initiated by addition of enzyme at 25℃ and the rate was followed at 412 nm.
Total RNA from homogenates of frozen liver was isolated using Trizol reagents, quantified by Nano drop, and stored at -70℃ prior to use. Relative quantification of expression of selected genes was performed using the Exicycler 96 Real-Time PCR System (Bioneer, Korea) and SYBR (Takara, Japan) as DNA binding dye for the detection of PCR products. The primer pairs for PCR were as follows : for FAS, forward 5'- GAT CCT GGA ACG AGA ACA CGA T-3', reverse 5'- GCC CAT TCG TAC ACG TCA TTC-3'; for HMG-CoA reductase (HMG-CR), forward 5'-; for acetyl-CoA carboxylase (ACC), forward 5'-; for sterol regulatory element binding protein 1c (SREBP1c), forward 5'- CCA CCG TCA CTT CCA GCT AGA C-3', reverse 5'- GTC GGC ATG GTC CTG ATT G-3'. The cycling conditions were 95℃ for 10 min, followed by 50 cycles of 95℃ for 10 sec and 60℃ for 1 min. To detect and eliminate possible primerdimer artifacts, the dissociation curve was generated by adding a cycle of 95℃ for 15 sec, 60℃ for 1 min and 95℃ for 15 sec. All primer sets produced amplicons of the expected size and their identity was also verified by sequencing. Results were normalized using the reference gene 18s RNA and represented as fold changes for reference gene.
Liver was removed from the mice and fixed in a buffer solution of 10% formalin. Fixed tissue was washed with running tap water for 6 h. After washing, the tissue was embedded in Paraffin block on tissue embedding center (Tissue-Tek, Sakura Finetek Co., Ltd, Japan), frozen, and stored at -20℃. The paraffin-embedded tissue block was sectioned at 5-um thickness on a microtome (Accu-Cut, Sakura Finetek Co., Ltd, Japan) and stained with haematoxylin for nuclear staining and eosin for cytoplasmic staining (H&E); stained areas were viewed using an optical microscope with a magnifying power of ×200.
The effect of MSW on the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical was estimated according to the method of Yoshikawa et al. [19] with a minor modification. Due to odd electron of DPPH, it presents strong absorption maximum at 517 nm. As this electron becomes paired off in the presence of a hydrogen donor such as antioxidants, the absorption intensity is reduced and the resulting decolorization is stoichiometric with respect to the number of electrons captured [20]. The 900 µl of MSW were mixed with 300 µl of DPPH solution (0.1 mM) and vortexed. The mixture was incubated at 37℃ for 30 min. The DPPH radical scavenging activity of MSW was calculated using the following equation: radical scavenging activity (%) = [(A control - A sample) / A control] × 100, where A sample is the absorbance of the sample and A control is the absorbance of the blank at 517 nm.
HepG2 cells (human hepatoma cell-line) were cultured in DMEM medium (Hyclone, USA) containing 10% fetal bovine serum supplemented with 100 U/ ml of penicillin, and 100 µg / ml of streptomycin (Gibco BRL, USA) at 37℃ in 5% CO2 atmosphere. After 24 h of incubation, HepG2 cells were treated with MSW (10 µl / ml) for 2 h, and the oxidative stress was induced by medium replacement with 0.5 mM concentration of t-BHP for 1 h. The fluorescent probe, dichlorofluorescein diacetate (DCF-DA), was used to monitor the intracellular generation of ROS induced by t-BHP. After 30 min of DCF-DA (50 uM) treatment, the intracellular ROS was monitored using a fluorescence microscope (Nikon, Eclipse TE2000-U, Japan).
HepG2 cells were treated with MSW for 2 h. The cells were then washed with phosphate-buffered saline (PBS), harvested in buffer containing 25 mM Tris-HCl (pH 7.4) and 125 mM sucrose by scraping, and centrifuged at 115 g for 10 min. The pellet was resuspended in PBS, sonicated for 15 seconds and centrifuged at 15,000 g for 20 min at 4℃. The supernatants were kept at -70℃ until analysis and protein content of supernatants was determined by the method of Bradford using bovine serum albumin as the standard. The catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), superoxide dismutase (SOD) activities were determined by using previously reported methods with slight modifications [21-24].
All data are represented as the mean ± SEM. Statistically significant differences between the groups were determined using student's t-test and in the case of DPPH assay, one-way ANOVA was applied and Tukey-Kramer test was used to compare the effects of diluted MSW versus the control TW on radical scavenging activity. P < 0.05 was considered to be significant.
All mice had free access to both food and water (TW or MSW) during the experimental period. The body weights appeared to be increased with time course in both groups. There were no statistically significant differences in the body weight and water intake between the TW and MSW group (Fig. 1).
Although there were no remarkable differences, plasma triglyceride (TG) and total cholesterol (TC) concentrations were slightly decreased in the MSW group. Feeding the high fat diet caused fatty liver with accumulation of TG and TC. The MSW group tended to have the loss of hepatic TG levels compared with control group at 10 weeks. The MSW administration attenuated the increase in hepatic TG due to the HFD (P < 0.05). Similarly, hepatic TC levels of MSW group were lowered up to about 35% compared to control group (Fig. 2).
No visible gross changes such as swelling or bleeding were observed in any organ or tissue at necropsy at 10 weeks. Photomicrographs of the H&E-stained liver at 10 weeks are shown in Fig. 3. In the liver histology, the liver of mice administered TW developed moderate hepatic steatosis. The hepacocytes were occupied by high accumulation of microvesicular- type fat in the cytoplasm and showed the ballooning character. In contrast with TW group, MSW group hardly showed recognizable fatty change of hepatocytes.
To investigate the effect of MSW on lipid metabolism, hepatic expressions of lipogenic genes were analyzed by real-time PCR (Fig. 4). The genes encoding lipogenic enzymes and transcription factors such as FAS, HMG-CR, ACC and SREBP1c were 42, 37, 24, and 33% lower, respectively, in the MSW group than in those of TW group.
Representative enzymes mediating lipid biogenesis and energy expenditure, the activities of FAS and CPT were assessed to investigate whether the MSW would affect lipid metabolism in the liver. Although no significant changes were found between groups in the present study, the activity of hepatic cytosolic FAS was significantly lower in the MSW group than in the TW group, while the activity of hepatic mitochondrial CPT in MSW group was higher than that of TW group (Fig. 5).
It has been known that antioxidants can neutralize free radicals by transferring either electrons or hydrogen atoms to DPPH which has been being used as a useful reagent for investigating free radical scavenging activities of tested materials [25]. If there is an antioxidant activity of tested material, the purple color of DPPH becomes yellowish. The treatment of MSW showed a significant color change indicating DPPH radical scavenging activity (P < 0.05), whereas no color changes were found in TW group. Moreover, this MSW activity supported with the color change was decreased by two-fold serial dilution with TW, which suggested that MSW had a free radical-scavenging activity (Fig. 6).
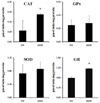
DCF-DA staining was applied to examine whether MSW was able to inhibit the ROS production in t-BHP-treated HepG2 cells, and the decreased fluorescence intensity was observed in MSW-treated HepG2 cells by fluorescence microscopy compared to TW-treated group (Fig. 7). As shown in Fig. 8, moreover, in measurement of antioxidant enzyme activities in t-BHP-treated HepG2 cells with MSW or TW, the CAT and GR activities of MSW group have shown a significant increase compared to TW group (P < 0.05). Although there were no significant differences, the GPx and SOD activities were slightly increased in MSW group compared to TW control. These findings were considered to reflect that MSW has an antioxidant effect in t-BHP-induced HepG2 cells.
The present study has shown that MSW, desalinated underground seawater collected from Jeju Island in Korea, improved lipid metabolism in HFD-fed C57BL/6 mice, as supported by modulating of lipid regulatory enzymes and histological findings. Moreover, MSW prevented ROS production, probably by the increased activities of antioxidant enzymes, in t-BHP-treated human hepatoma cell line, HepG2 cells.
Although the body weights and plasma lipid levels were not different between groups, MSW decreased hepatic lipid levels after 10 weeks of administration. These results suggest that suppressed lipid accumulation was involved in preventing fatty liver in MSW-treated mice. Fatty liver generally results from hepatic lipid accumulation as a result of an increase in the supply of circulating free fatty acids to the liver, as well as increased endogenous hepatic FAS, a major enzyme that catalyzes the synthesis of saturated long-chain fatty acid [26]. In this study, the MSW inhibited the activity of FAS compared to the control group. In contrast, the increased activity of CPT, which is the rate-limiting enzyme that facilitates the entry of fatty acids into the mitochondria for oxidation [27], was found in MSW group. In addition, the histological observation on liver tissues between groups showed that the accumulation of fats in the liver was clearly blocked by MSW treatment. Thus, it was suggested that MSW might prevent the development of fatty liver by modulation of hepatic lipid-regulating enzyme actions. To further investigate the reduced hepatic lipid accumulation in MSW-treated mice, we explored the mRNA expression of lipogenic genes, including FAS, ACC1, HMG-CR, and SREBP1c, using quantitative real-time PCR. SREBP1c is a key transcription factor regulating the expression of enzymes involved in lipogenesis and fatty acid desaturation, as well as in response to feeding and insulin [28,29]. In this study, suppression of SREBP1c mRNA by MSW administration was confirmed, which in turn would be expected to down-regulate lipogenic genes such as FAS and ACC. As might be expected, mRNA expression of these lipogenic genes was decreased in the present study. Hepatic mRNA expression level of HMG-CR, which is a rate-limiting enzyme in the de novo synthesis of cholesterol [30], was decreased in the MSW-treated mice as well. From these results, we suggest that the suppression of hepatic fat accumulation by MSW in HFD-fed mice may be mediated by coordinated action on hepatic lipid-regulating enzyme action and by down-regulating expression of lipogenic genes. Several studies demonstrated inhibition effects of Ca on lipid accumulation in plasma and tissues [31,32]. Moreover, Hwang et al. [8] reported the anti-obesity effects of deep sea water in ob/ob mice recently and speculated that Ca contained in deep sea water could be involved in these effects. An exact molecular mechanism on relationship between MSW and improved lipid metabolism is not clarified in this study. However, Ca contained in MSW could have effects on lipid metabolism, partially.
Along with manganese and chloride, Mg is a cofactor for some 300 enzymes in the body and acts in concert with Ca to support cell, tissue, and organ functions [33]. Mg deficiency and oxidative stress have both been identified as pathogenic factors in several metabolic diseases. The link between these two factors is unclear in humans although, in experimental animals, severe Mg deficiency has been shown to lead to increased oxidative stress [34,35]. Therefore, the present study was also carried out to investigate the effect of MSW on antioxidant systems in oxidative stress induced HepG2 cells by t-BHP. DPPH radical scavenging assay has been widely used for screening antioxidant potential of various kinds of bio-molecules and natural products [36,37]. In this study, the treatment of MSW showed a significant color change indicating DPPH radical scavenging activity, which suggested that MSW had a free radical-scavenging activity. It has been known that the pro-oxidant such as t-BHP can directly oxidize DCF-DA to fluorescent DCF. Also, it can decompose peroxyl radicals and generate ROS and lipid peroxide, thus increasing fluorescence [38]. In the present study, MSW has shown an effective ability to quench DPPH radical and a decreased intensity of fluorescence in t-BHP- and DCF-DA-treated HepG2 cells. From these findings, it was presumed that the activities of intracellular antioxidant enzymes such as CAT, GPx, SOD, and GR played an important role to defense against t-BHP-induced oxidative stress in HepG2 cells. Indeed, MSW significantly increased CAT and GR activities in t-BHP-treated HepG2 cells compared to TW control group, and although it was not significantly different, SOD and GPx activities were enhanced in MSW group as well. Because change of these enzyme activities can be considered as a biomarker of antioxidant response under the condition of oxidative stress [39], the increased and enhanced activities of antioxidant enzymes determined in MSW-treated HepG2 cells strongly suggested that MSW had an ROS dissipating effect against experimentally induced-oxidative stresses.
Previously, the desalinated deep seawater collected in Kochi area of Japan was reported to significantly increase the plasma GPx activity in dietary induced hyperlipidemic rabbit model, and the desalinated deep seawater also showed increased plasma SOD activity compared to control group [5]. Although the experimental conditions are different in both studies, it should be noted that the common feature is the improvement of antioxidant activities that confers the beneficial effects in experimentally induced atherosclerosis and acute liver injury model.
In conclusion, the present study demonstrates that the desalinated underground seawater of Jeju Island in Korea, MSW, has a fatty liver preventative effect which appears to be partly mediated by suppressing hepatic fat accumulation by down-regulation of genes expression related with lipogenesis, along with inhibition of fatty acid formation through FAS in HFD-fed C57BL/6 mice. On the other hand, MSW has a suppression effect on ROS production induced oxidative stress by t-BHP-treatment in HepG2 cells. Presumably, as a possible mechanism contributing to these effects, MSW seems to increase the intracellular activities of antioxidant enzymes and in turn to enhance the quenching of free radicals. These results suggest that MSW can play an important role in regulating the lipid and antioxidant metabolism. However, further studies are needed to elucidate the precise underlying mechanism of lipid metabolism and antioxidant activities of MSW. To our knowledge, this study is the first report to evaluate the usefulness of the desalinated underground seawater, and it seems that more information will be available in the future as further studies are completed.
Figures and Tables
Fig. 1
Weekly changes of body weight and water intake in C57BL/6 mice treated with high fat diet (HFD) and drinking of desalinated underground seawater. Values are mean ± SEM (n=10, each group).
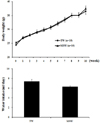
Fig. 2
Plasma and hepatic lipid concentrations determined in C57BL/6 mice treated with high fat diet (HFD) and drinking of desalinated underground seawater. Value are mean ± SEM. *Significantly different from tap water control group at P < 0.05 (student's t-test). MSW: magma seawater (desalinated underground seawater), TW: tap water.

Fig. 3
Representative photomicrographs showing inhibited fatty liver in C57BL/6 mice treated with high fat diet (HFD) and drinking of desalinated underground seawater. Values are mean ± SEM (n=5, each group). MSW: magma seawater (desalinated underground seawater), TW: tap water.
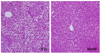
Fig. 4
Quantitative mRNA expressions of liver lipogenesis genes in C57BL/6 mice treated with high fat diet (HFD) and drinking of desalinated underground seawater. Values are mean ± SEM. The normalization of the gene was carried out 18s RNA expression for each group. FAS: fatty acid synthase, HMG-CR: -hydroxy-3-methyl-glutaryl-CoA reductase, ACC: acetyl-CoA carboxylase, SREBP1c: sterol regulatory element binding protein 1c. MSW: magma seawater (desalinated underground seawater), TW: tap water.

Fig. 5
Activities of hepatic lipid regulating enzymes in C57BL/6 mice treated with high fat diet (HFD) and drinking of desalinated underground seawater. Values are mean ± SEM. *Significantly different from tap water control group at P < 0.05 (student's t-test). FAS: fatty acid synthase, CPT: carnitine palmitoyltransferase. MSW: magma seawater (desalinated underground seawater), TW: tap water.

Fig. 6
Effect of desalinated underground seawater on DPPH radical scavenging activity. The relative DPPH scavenging activities of desalinated underground seawater to the tap water group are expressed as percentage values (mean ± SEM.) of triplicated determinations, and bars with different letters indicate significant differences at P < 0.05 (one-way ANOVA) among groups. Fraction numbers represent two-fold serial dilutions of desalinated underground seawater. MSW: magma seawater (desalinated underground seawater), TW: tap water.

Fig. 7
Represntative fluorescence microscopic findings on ROS production induced by t-BHP in desalinated underground seawater-treated HepG2 cells. Reduced fluorescence intensity was observed in desalinated underground seawater group. MSW: magma seawater (desalinated underground seawater), TW: tap water. DCF-DA (2'-7'-dichlorofluorescein diacetate) staining, magnification × 200.
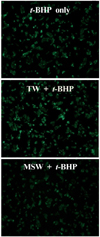
Fig. 8
Effect of desalinated underground seawater effect on antioxidant enzyme activities in t-BHP-treated HepG2 cells. Intracellular antioxidant enzyme activities were represented as mean ± SEM. of triplicated determination. *Significantly different from tap water control group at P < 0.05 (student's t-test). CAT: catalase, SOD: superoxide dismutase, GPx: glutathione peroxidase, GR: glutathione reductase, MSW: magma seawater (desalinated underground seawater), TW: tap water.
References
1. Hataguchi Y, Tai H, Nakajima H, Kimata H. Drinking deep-seawater restores mineral imbalance in atopic eczema/dermatitis syndrome. Eur J Clin Nutr. 2005. 59:1093–1096.

2. Nakasone T, Akeda S. The application of deep sea water in Japan. UJNR Technical Report. 1999. 28:69–75.
3. Aoyama M, Hirose K, Miyao T, Igarashi Y. Low level 137Cs measurements in deep seawater samples. Appl Radiat Isot. 2000. 53:159–162.

4. Katsuda S, Nakagawa K, Miyake M, Yamasaki M, Katahira K, Mohri M, Shimizu T, Hazama A. Deep-seawater improves cardiovascular hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol Pharm Bull. 2008. 31:38–44.

5. Miyamura M, Yoshioka S, Hamada A, Takuma D, Yokota J, Kusunose M, Kyotani S, Kawakita H, Odani K, Tsutsu Y, Nishioka Y. Difference between deep seawater and surface seawater in the preventive effect of atherosclerosis. Biol Pharm Bull. 2004. 27:1784–1787.

6. Tsuchiya Y, Watanabe A, Fujisawa N, Kaneko T, Ishizu T, Fujimoto T, Nakamura K, Yamamoto M. Effects of Desalted Deep Seawater on Hematologic and Blood Chemical Values in Mice. Tohoku J Exp Med. 2004. 203:175–182.

7. Yoshioka S, Hamada A, Cui T, Yokota J, Yamamoto S, Kusunose M, Miyamura M, Kyotani S, Kaneda R, Tsutsui Y, Odani K, Odani I, Nishioka Y. Pharmacological activity of deep-sea water: examination of hyperlipemia prevention and medical treatment effect. Biol Pharm Bull. 2003. 26:1552–1559.

8. Hwang HS, Kim SH, Yoo YG, Chu YS, Shon YH, Nam KS, Yun JW. Inhibitory effect of deep sea water on differentiation of 3T3-L1 adipocytes. Mar Biotechnol (NY). 2008. 11:161–168.

9. Bloomgarden ZT. Magnesium deficiency, atherosclerosis, and health care. Diabetes Care. 1995. 18:1623–1627.
10. Robertson DS. Magnesium or calcium hypophosphite could be a treatment for obesity in humans. Med Hypotheses. 2006. 66:439–440.

11. Rude RK. Magnesium deficiency and diabetes mellitus. Causes and effects. Postgrad Med. 1992. 92:217–219. 222–224.
12. Huang QL, Jin Y, Zhang LN, Cheung PCK, Kennedy JF. Structure, molecular size and antitumor activities of polysaccharides from Poria cocos mycelia produced in fermenter. Carbohydr Polym. 2007. 70:324–333.

13. Ibrahim W, Lee US, Yeh CC, Szabo J, Bruckner G, Chow CK. Oxidative stress and antioxidant status in mouse liver: effects of dietary lipid, vitamin E and iron. J Nutr. 1997. 127:1401–1406.

14. Mursu J, Voutilainen S, Nurmi T, Rissanen TH, Virtanen JK, Kaikkonen J, Nyyssönen K, Salonen JT. Dark Chocolate Consumption Increases HDL Cholesterol Concentration and Chocolate Fatty Acids May Inhibit Lipid Peroxidation in Healthy Humans. Free Radic Biol Med. 2004. 37:1351–1359.

15. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959. 37:911–917.

16. Hulcher PH, Oleson WH. Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl CoA reductase by measurement of coenzyme A. J Lipid Res. 1973. 14:625–631.

17. Nepokroeff CM, Lakshmanan MR, Porter JW. Fatty-acid synthase from rat liver. Meth Enzymol. 1975. 35:37–44.
18. Markwell MA, McGroarty EJ, Bieber LL, Tolbert NE. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J Biol Chem. 1973. 248:3426–3432.

19. Yoshikawa T, Naito Y, Kondo M. Hiramatsu M, Yoshikawa T, Inoue M, editors. Food and diseases 2. Free radicals and diseases. 1997. New York: 11–19.
20. Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of food by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem. 1998. 62:1201–1204.

21. Aebi H. Bergmeyer HU, editor. Catalase. Methods of enzymatic analysis. 1974. . New York: Academic press;673–684.

22. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974. 47:469–474.

23. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967. 70:158–169.
24. Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969. 112:109–115.

25. Naik GH, Priyadarsini KI, Satav JG, Banavalikar MM, Sohoni DP, Biyani MK, Mohan H. Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry. 2003. 63:97–104.

26. Latasa MJ, Griffin MJ, Noon YS, Kang CH, Sul HS. Occupancy and function of the 150 sterol regulatory element and -65 E-box in nutritional regulation of the fatty acid synthase gene in living animals. Mol Cell Biol. 2003. 23:5896–5907.

27. McGarry JD, Brown NF. The mitochondrial carnitine palmitoy-ltransferase system. From concept to molecular analysis. Eur J Biochem. 1997. 244:1–14.

28. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002. 109:1125–1131.

29. Paulauskis JD, Sul HS. Hormonal regulation of mouse fatty acid synthase gene transcription in liver. J Biol Chem. 1989. 264:574–577.

30. Hwa JJ, Zollman S, Warden CH, Taylor BA, Edwards PA, Fogelman AM, Lusis AJ. Genetic and dietary interactions in the regulation of HMG-CoA reductase gene expression. J Lipid Res. 1992. 33:711–725.

31. Iacono JM. Effect of varying the dietary level of calcium on plasma and tissue lipids of rabbits. J Nutr. 1974. 104:1165–1171.

32. Yacowitz H, Fleischman AI, Amsden RT, Bierenbaum ML. Effects of dietary calcium upon lipid metabolism in rats fed saturated or unsaturated fat. J Nutr. 1967. 92:389–392.

33. Lukaski HC, Nielsen FH. Dietary magnesium depletion affects metabolic responses during submaximal exercise in postmenopausal women. J Nutr. 2007. 132:930–935.

34. Garcia LA, Dejong SC, Martin SM, Smith RS, Buttner GR, Kerber RE. Magnesium reduces free radicals in an in vivo coronary occlusion-reperfusion model. J Am Coll Cardiol. 1998. 32:536–539.

35. Kumar BP, Shivakumar K. Depressed antioxidant defense in rat heart in experimental magnesium deficiency. Implications for the pathogenesis of myocardial lesions. Biol Trace Elem Res. 1997. 60:139–144.

36. Fagali N, Catalá A. Antioxidant activity of conjugated linoleic acid isomers, linoleic acid and its methyl ester determined by photoemission and DPPH techniques. Biophys Chem. 2008. 137:56–62.

37. Hirano R, Sasamoto W, Matsumoto A, Itakura H, Igarashi O, Kondo K. Antioxidant ability of various flavonoids against DPPH radicals and LDL oxidation. J Nutr Sci Vitaminol (Tokyo). 2001. 47:357–362.

38. Martín MA, Ramos S, Mateos R, Granado Serrano AB, Izquierdo-Pulido M, Bravo L, Goya L. Protection of human HepG2 cells against oxidative stress by cocoa phenolic extract. J Agric Food Chem. 2008. 56:7765–7772.

39. Sies H. Strategies of antioxidative defense. Eur J Biochem. 1993. 215:213–219.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download