Abstract
The apoptotic effect of bacteria-derived β-glucan was investigated in human colon cancer cells SNU-C4 using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay, reverse transcription-polymerase chain reaction (RT-PCR) expressions of Bcl-2, Bax, and Caspase-3 genes, and assay of caspase-3 enzyme activity. β-Glucan of 10, 50, and 100 µg/mL decreased cell viability in a dose-dependent manner with typical apoptotic characteristics, such as morphological changes of chromatin condensation and apoptotic body formation from TUNEL assay. In addition, β-glucan (100 µg/mL) decreased the expression of Bcl-2 by 0.6 times, whereas the expression of Bax and Caspase-3 were increased by 3.1 and 2.3 times, respectively, compared to untreated control group. Furthermore, the caspase-3 activity in the β-glucan-treated group was significantly increased compared to those in control group (P < 0.05). Bacterial derived β-glucan could be used as an effective compound inducing apoptosis in human colon cancer.
Generally, β-glucan, which has β-1,3 and 1,4 glycosides linkage of D-glucose, was derived from oats (Behall et al., 1997; Davidson et al, 1991), barley (McIntosh et al., 1991) or yeast (Nicolosi et al., 1999). β-Glucan have been reported to inhibit the growth of bacteria, virus, and fungus (Nameda et al., 2007), to stimulate macrophages as immune enhancer (Volman et al., 2008), and to reduce cholesterol level (Newman et al., 1989) and glucose concentration (Hong et al., 2005) in the blood. Several mechanisms have been proposed for the protective and health beneficial effects of β-glucan including antioxidant capacity (Novak & Vetvicka, 2008; Ohno et al., 1996) and functional properties as dietary fiber source.
β-Glucan can be derived from Alcaligenes and Agrobacterium species and their β-glucan has a homopolymeric structure with linear β-1,3-glucosidic linkage (Hong et al., 2005). Physiological effects of bacteria-derived β-glucan including anti-cancer activity has not been reported in the literature. Enhancing apoptosis of cancer cells is one of representative methods for searching anti-cancer candidates.
Apoptosis, known as programmed cell death, is an essential physiologic process in the normal development for maintaining cell homeostasis. Therapeutic strategy for cancer is induction of apoptosis, and many cancers are underlined by the identification of many oncogenes as regulators of apoptosis such as Bcl-2. Bcl-2 was the first isolated protein that regulated the life or death of a cell through controlling the release of mitochondrial apoptogenic factors that are associated with death protease called caspases (Oltvai et al., 1993). For these reasons, apoptosis has been a very essential event for the researchers who seek new strategies against cancer. Although many studies on the anti-tumor effects of β-glucan have been reported, anti-colon cancer mechanism of β-glucan through caspase pathway has not fully understood.
Therefore, the objective of this study was to examine apoptotic effects of bacteria-derived β-glucan on human colon cancer cells SNU-C4 through caspase-3 pathway.
β-Glucan produced by fermentation with a mutant strain of Aureobasidum species was obtained from Korea Research Institute of Bioscience and Biotechnology (Jeongeup, Korea). The structure of β-glucan produced by Aureobasidum species was a homopolymer with β-1,3 glycosidic bond, which had high viscosity and solubility (Hong et al., 2005).
Human colon cancer SNU-C4 cells were obtained from Korean Cell Line Bank (KCLB, Seoul, Korea). Cells were maintained in RPMI 1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA). Cells were grown in a humidified incubator at 37℃ in an atmosphere of 5% CO2 and 95% air.
Cytotoxicity was performed by MTT assay (Sigma, St. Louis, MO, USA) as previously described (Park et al., 2008). In brief, SNU-C4 cells were cultured in each well of a 96-well plate (Coring Incorporated, Coring, NY, USA). The β-glucan was added to the cells with the concentrations of 1, 5, 10, 20, 25, 50, 100, and 200 µg/mL at 24 hr. Control was the cells without addition of β-glucan. After a further 24 hrs incubation, 20 µL of MTT was added to each well and incubated for 4 hr. After the plate was incubated in the dark for 4 hr, the medium was discarded and 150 µL of DMSO was added to each well and incubated for 20 min. The absorbance at a test wavelength of 540 nm with a reference wavelength of 690 nm was measured using a microtiter plate reader (Molecular Devices, CA, USA). The optical density (O.D.) was calculated as the difference between the absorbance from reference wavelength and from test wavelength. Percent viability was calculated as (O.D. of β-glucan treated sample / O.D. of none treated sample) ×100.
Cell samples were fixed using a freshly prepared solution (4% paraformaldehyde in PBS, pH 7.4) for 1 hr at 15-25℃ and incubated in permeabilization solution (0.1% Trixon X-100 in 0.1% sodium citrate) for 2 min on ice. Then a 50 µL TUNEL (Roche) reaction mixture was added to samples and incubated for 60 min at 37℃ in a humidified atmosphere in the dark. After converted-POD (antibody conjugated with peroxidase) solution was added, the cells were incubated for 30 min. The DNA fragments stained using 3, 3'-diaminobenzidine (Sigma, St. Louis, MO, USA) were used as substrates for the peroxidase.
The procedures of RT-PCR were the same as in the previous study (Park et al., 2008). Briefly, total RNA of cells was extracted by Trizol (Invitrogen, Carlsbad, CA, USA). cDNA synthesis was performed in 20 µL reaction system of reverse transcription including 2 µg RNA. The expression of apoptosis-related genes, Bcl-2, Bax and Caspase-3, was determined by RT-PCR. PCR was performed with the following primers for Bcl-2 (5'-TCC GTG CCT GAC TTT AGC AAG CTG-3' and 5'-GGA ATC CCA ACC AGA GAT CTC AA-3'), for Bax (5'-AGA TGA ACT GGA TAG CAA TAT GGA-3' and 5'-CCA CCC TGG TCT TGG ATC CAG ACA-3') and for Caspase-3 (5'-CTT GGT AGA TCG GCC ATC TGA AAC-3' and 5'-GGT CCC GTA CAG GTG TGC TTC GAC-3'). The Cyclophilin (5'-ACC CCA CCG TGT TCT TCG AC-3' and 5'-CAT TTG CCA TGG ACA AGA TG-3') was used as an internal control. The annealing temperature was 50℃ for Bcl-2 and Bax, 57℃ for Caspase-3, and 56℃ for Cyclophilin. The amplified fragment sizes of Bcl-2, Bax, Caspase-3, and Cyclophilin were 333-bp, 260-bp, 405-bp and 300-bp, respectively. PCR products were separated by 2% agarose gel electrophoresis, then scanned and analyzed by image analyzer.
Caspase-3 activity was measured with a caspase-3 assay kit (Sigma, St. Louis, MO, USA) according to the manufacturer's protocol. SNU-C4 cells were lysed with lysis buffer after treatment with β-glucan (50 and 100 µg/mL) for 24 hr. Caspase-3 was used as a positive control for comparison. All mixtures were incubated overnight in a humidified environment at 37℃. The concentration of the p-nitroanyline (p-NA) released from the substrate was measured at 405 nm.
Dosage effects of β-glucan on the viability of SNU-C4 cells are shown in Figure 1. β-Glucan treatment displayed a significant dose-dependent decrease in cell viability (P < 0.05). Viability of cells treated with β-glucan at the concentrations of 0, 1, 5, 10, 20, 25, 50, 75, 100, 150, and 200 µg/mL for 24 hr was 100.0 ± 1.7, 90.7 ± 0.6, 83.7 ± 2.5, 63.4 ± 3.6, 62.2 ± 1.1, 63.0 ± 3.3, 60.6 ± 0.9, 59.7 ± 1.0, 57.2 ± 1.5, 52.6 ± 1.2, and 44.7 ± 1.6% of control, respectively (Fig. 1). This result indicates that β-glucan induced cell death in SNU-C4 cells with a dose-dependent manner. For further experiment, concentrations of 10, 50, and 100 µg/mL β-glucan were selected, which showed lower cell toxicity than 45%. Also, these concentrations have significantly higher cell toxicity than 0, 1, and 5 µg/mL and lower cell toxicity than 200 µg/mL.
During the process of apoptosis in cells, DNA strand breaks occur and the nicks in DNA molecules can be quantitatively measured (Fig. 2A-B, C, and D). Through TUNEL in situ detection, we found that apoptotic cells were increased in SNU-C4 cells after 24 hr treatment with 10, 50, and 100 µg/mL (Fig. 2B-F, G, and H), compared to control without β-glucan treatment (Fig. 2B-E). The results of TUNEL assay can provide early phenomenon for apoptosis as the loss of mitochondrial membrane potential (Moudy et al., 1995; Smiley et al., 1991).
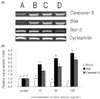
RT-PCR analysis of the mRNA levels of Bcl-2, Bax, and Caspase-3 was performed to estimate the expression of apoptosis-related genes (Fig. 3A). The expression of Bcl-2 was decreased significantly by 0.5 and 0.6 times in β-glucan treated with 50 and 100 µg/mL respectively, compared to that of control (P < 0.05) (Fig. 3B). Also, the effect of β-glucan treatment on the expression of Bax gene was increased significantly by 1.7, 2.5, and 3.1 times in β-glucan treated with 10, 50, and 100 µg/mL, respectively, compared to control (P < 0.05) (Fig. 3B). Finally, gene expression of Caspase-3 was increased significantly by 1.5, and 2.3 times in β-glucan treated with 50, and 100 µg/mL, respectively, compared to control (P < 0.05) (Fig. 3B). The most effective concentrations for the expression of these genes in SNU-C4 cells were 50 and 100 µg/mL of β-glucan.
Caspase-3 enzyme activity in 50 and 100 µg/mL β-glucan treated cells is shown in Fig. 4. The caspase-3 activities in SNU-C4 cells treated with 50 and 100 µg/mL were significantly higher than those of SNU-C4 cells without β-glucan (P < 0.05) while significantly lower than caspase-3 positive cells. However, caspase-3 activity of β-glucan treated cells between 50 and 100 µg/mL was not significant.
The present study has demonstrated that β-glucan induced apoptosis in human colon cancer SNU-C4 cells. Addition of β-glucan inhibited cell proliferation, induced apoptosis, led to changes of cell morphology and of the expression of apoptotic genes, and increased the activity of caspase-3 enzyme. Yamamoto et al. (2009) reported that β-glucan from mushroom induced suppression of angiogenesis and metastasis in orally administrated animal models. Kobayashi et al. (2005) demonstrated that the β-glucan from Agaricus blazei Murill reduced pulmonary metastasis of 3LL cells and peritoneal disseminated metastasis of HRA cells and inhibited the growth of these metastatic tumors in the lung. Previous studies reported that β-glucan has the ability of a potential anticancer agent in vitro and in vivo animal models.
The results of this study suggested that β-glucan could induce apoptosis in human colon cancer cells. Furthermore, the results confirmed possible apoptosis inducing mechanisms of the β-glucan through TUNEL assay, gene expressions related to apoptosis, and caspase-3 activity. Based on these results, β-glucan induced apoptosis of human colon cancer cells at the concentration of 100 µg/mL. These cells undergoing apoptosis exhibit cytoplasmic blebbing, nuclear shrinkage, chromatin condensation, irregularity in shape and retraction (Cohen, 1993; Qiao et al., 1998). These characteristic changes were observed in the morphology of cells treated with 50 and 100 µg/mL β-glucan. RT-PCR analysis of the mRNA levels of Bcl-2 showed significantly decrease, and those of Bax and Caspase-3 were increased significantly with β-glucan treatment (P < 0.05). As many cancer cells exhibit abnormality induced by target compounds, exploiting these alterations of regulators induced by natural sources could be an efficient tool to screen promising therapeutic compounds for cancer treatment (Harlozinska, 2005; Siripong et al., 2009). Numerous studies show that caspase-3 pathway has anti-apoptotic effects which have been implicated in a variety of biological processes (Yang et al., 2009). The caspase-3 activity in the β-glucan treated group was significantly increased, compared to that in control group, implying that β-glucan can induce apoptosis in cancer cells through caspase-3 pathway.
In conclusion, the induction of apoptosis by β-glucan in colon cancer cells may be related with modulation of Bcl-2 family resulting in the activation of caspase-3 expression. It may be a beneficial natural agent for colon cancer treatment and chemoprevention.
Figures and Tables
Fig. 1
Cell viability of β-glucan in human colon cancer cells SNU-C4 for 24 hrs. Viability was determined with MTT assay. Results are presented as mean ± SEM. The experiments were done triplicate. Different letters denote significant differences within each group (P < 0.05).

Fig. 2
Morphological change of β-glucan induced cell death in human colon cancer cells SNU-C4. Cells were cultured without resveratrol (A) or with 10, 50, and 100 µg/mL of β-glucan (B, C, and D). Morphology (top): phase-contrast microscopy showed cell shrinkage, irregularity of shape in β-glucan treated cultures. TUNEL assay (bottom): SNU-668 cells stained using TUNEL method. Condensed and marginated chromatin showed to be stained dark brown. The experiments were done triplicate. Scale bar, 100 µm.

Fig. 3
RT-PCR analysis of Bcl-2, Bax and Caspase-3. As the internal control, Cyclophilin mRNA was also β-glucan transcribed. A: control, B: 10 µg/mL of β-glucan treated group, C: 50 µg/mL of β-glucan treated group, D: 100 µg/mL of β-glucan treated group. The experiments were done triplicate. Different letters denote significant differences within each group (P < 0.05).
References
1. Behall KM, Scholfield DJ, Hallfrisch J. Effect of β-glucan level in oat fiber extracts on blood lipids in men and women. J Am Coll Nutr. 1997. 16:46–51.

3. Davidson MH, Dugan LD, Burns JH, Bova J, Story K, Drennan KB. The ypocholesterolemic effects of β-glucan in oatmeal and oat bran: a dose-controlled study. JAMA. 1991. 265:1833–1839.

4. Harlozinska A. Progress in molecular mechanisms of tumor metastasis and angiogenesis. Anticancer Res. 2005. 25:3327–3333.
5. Hong KH, Jang KH, Lee JC, Kim SH, Kim MK, Lee IY, Kim SM, Lim YH, Kang SA. Bacterial β-glucan exhibits potent hypoglycemic activity via decrease of serum lipids and adiposity, and increase of UCP mRNA expression. J Microbiol Biotechnol. 2005. 15:823–830.
6. Kobayashi H, Yoshida R, Kanada Y, Fukuda Y, Yagyu T, Inagaki K, Kondo T, Kurita N, Suzuki M, Kanayama N, Terao T. Suppressing effects of daily oral supplementation of β-glucan extracted from Agaricus blazei Murill on spontaneous and peritoneal disseminated metastasis in mouse model. J Cancer Res Clin Oncol. 2005. 131:527–538.

7. McIntosh GH, Whyte J, McArthur R, Nestel PJ. Barley and wheat foods: influence on plasma cholesterol concentrations in hypercholesterolemic men. Am J Clin Nutr. 1991. 53:1205–1209.

8. Moudy AM, Handran SD, Goldberg MP, Ruffin N, Karl I, Kranz-Eble P, DeVivo DC, Rothman SM. Abnormal calcium homeostasis and mitochondrial polarization in a human encephalomyopathy. Proc Natl Acad Sci U S A. 1995. 92:729–733.

9. Nameda S, Miura NN, Adachi Y, Ohno N. Antibiotics protect against septic shock in mice administered β-glucan and indomethacin. Microbiol Immunol. 2007. 51:851–859.

10. Newman RK, Lewis SE, Newman CW, Bioik RJ, Ramage RT. Hypocholesterolemic effects of barley food on healthy men. Nutr Rep Int. 1989. 34:749–760.
11. Nicolosi R, Bell SJ, Bistrian BR, Greenberg I, Forse RA, Blackburn GL. Plasma lipid changes after supplementation with β-glucan fiber from yeast. Am J Clin Nutr. 1999. 70:208–212.

12. Novak M, Vetvicka V. β-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J Immunotoxicol. 2008. 5:47–57.

13. Ohno N, Egawa Y, Hashimoto T, Adachi Y, Yadomae T. Effect of β-glucans on the nitric oxide synthesis by peritoneal macrophage in mice. Biol Pharm Bull. 1996. 19:608–612.

14. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993. 74:609–619.

15. Park HJ, Kim MJ, Ha E, Chung JH. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine. 2008. 15:147–151.

16. Qiao L, Hanif R, Sphicas E, Shiff SJ, Rigas B. Effect of aspirin on induction of apoptosis in HT-29 human colon adenocarcinoma cells. Biochem Pharmacol. 1998. 55:53–64.

17. Siripong P, Hahnvajanawong C, Yahuafai J, Piyaviriyakul S, Kanokmedhakul K, Kongkathip N, Ruchirawat S, Oku N. Induction of Apoptosis by Rhinacanthone isolated from Rhinacanthus nasutus roots in human cervical carcinoma cells. Biol Pharm Bull. 2009. 32:1251–1260.

18. Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD Jr, Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991. 88:3671–3675.

19. Volman JJ, Ramakers JD, Plat J. Dietary modulation of immune function by β-glucans. Physiol Behav. 2008. 94:276–284.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download