Abstract
BACKGROUND/OBJECTIVES
MATERIALS/METHODS
RESULTS
Figures and Tables
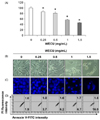 | Fig. 1Inhibition of cell viability and induction of apoptosis by WECU in MDA-MB-231 cells.MDA-MB-231 cells were treated with various concentrations of WECU for 72 h. (A) The cell viability was measured by an MTT assay. The data were expressed as the means ± SD of three independent experiments (* P < 0.05 vs. untreated control). (B) The morphological changes of MDA-MB-231 cells treated with WECU in various concentrations were observed under an inverted microscope (magnification, ×200). (C) The cells were stained with DAPI, and then the nuclei were photographed with a fluorescence microscope using a blue filter (magnification, ×400). (D) The percentages of annexin V-FITC positive cells in the top (PI negative) and bottom (PI positive) right quadrant were indicated. Each point represents the means of two independent experiments.
|
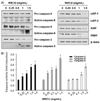 | Fig. 2Activation of caspases and inhibition of IAP family proteins expression by WECU in MDA-MB-231 cells.MDA-MB-231 were treated with the indicated concentrations of WECU for 72 h. (A) The equal amounts of cellular proteins were probed with the indicated antibodies, and the proteins were visualized using an ECL detection system. Actin was used as an internal control. (B) The activities of the caspases were evaluated using caspases assay kits. The data were expressed as the means ± SD of three independent experiments (* P < 0.05 vs. untreated control).
|
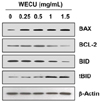 | Fig. 3Effects of WECU on the levels of BCL-2 family proteins in MDA-MB-231 cells.After 72 h incubation with the indicated concentrations of WECU, the cellular proteins were probed with anti-BAX, anti-BCL-2 and anti-BID antibodies. Equal protein loading was confirmed by analysis of actin in the protein extracts.
|
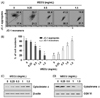 | Fig. 4Effects of WECU on the MMP values and cytochrome c expression in MDA-MB-231 cells.A and B) After 72 h incubation with the indicated concentrations of WECU, the changes in MMP were analyzed on a flow cytometer. (A) An example of representative results according to each treatment concentration is presented. (B) The data were expressed as the means ± SD of three independent experiments (* P < 0.05 vs. untreated control). (C and D) Cells cultured under the same conditions were lysed, and cytosolic (C) and mitochondrial (D) proteins were probed with an anti-cytochrome c antibody. Equal protein loading was confirmed by analysis of actin and cytochrome oxidase subunit VI (COX VI) in each protein extract.
|
 | Fig. 5Induction of ROS generation by WECU in MDA-MB-231 cells.(A) MDA-MB-231 cells were either treated with 1.5 mg/mL WECU for the indicated times or pre-treated with NAC (10 mM) for 1 h before WECU treatment. ROS generation was measured by a flow cytometer. The data are the means of the two different experiments. (B) The cells were either treated with 1.5 mgL WECU for 1 h or pre-treated with NAC (10 mM) for 1 h before WECU treatment and then stained with DCF-DA. Images were obtained using a fluorescence microscope (Original magnifications: 200×). The images presented here are captured from one experiment and are representative of at least three independent experiments.
|
 | Fig. 6Induction of ROS-dependent apoptosis by WECU in MDA-MB-231 cells.MDA-MB-231 cells were pre-treated with or without 10 mM NAC for 1 h, prior to 1.5 mg/mL WECU treatment. (A) After 72 h incubation, the cells were stained with DAPI, and then the nuclei were photographed (magnification, ×400). (B) The percentages of annexin V-FITC positive cells cultured under the same conditions in the top (PI negative) and bottom (PI positive) right quadrant were indicated. Each point represents the mean of two independent experiments. (C) Cell viability was analyzed using MTT assay. The data are expressed as means ± SD of three independent experiments (* P < 0.05 vs. untreated control; # P < 0.05 vs. WECU-treated cells).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download