Abbreviations
NAFLD
EAF
IR
TG
FFAs
VLDL
SREBP1c
FASN
ACLY
WD
FER
AST
ALT
ALP
Journal List > Nutr Res Pract > v.12(2) > 1050931
NAFLD
EAF
IR
TG
FFAs
VLDL
SREBP1c
FASN
ACLY
WD
FER
AST
ALT
ALP
 | Fig. 1Ethanol extract of Allium fistulosum (EAF) attenuates lipid accumulation in HepG2 cells.A) Non-cytotoxic concentrations of EAF significantly block oleic acid (OA)-mediated lipid accumulation. HepG2 cells treated in the presence or absence of OA, alone or in combination with EAF, were stained by using an Oil Red O solution. The values presented are mean ± SD for three independent experiments. Means not sharing a common superscript are significantly different, P < 0.05. (B) EAF at concentrations up to 400 µg/mL did not produce cytotoxicity in HepG2 cells. Cell viability was measured by using a WST-1 assay in HepG2 cells treated in the presence or absence of OA, alone or in combination with EAF, for 24 h. There was no statistical significance detected.
|
 | Fig. 2Ethanol extract of Allium fistulosum (EAF) inhibits transcriptional activity of lipogenesis-related genes.The mRNA expressions of genes associated with lipid metabolism were measured by performing qRT-PCR. EAF was treated alone or in combination at either 100 or 200 µg/mL in the presence or absence of oleic acid (OA) in HepG2 cells for 18 h. All experiments were conducted independently three times and data are expressed as mean ± SD. Means not sharing a common superscript are significantly different, P < 0.05. SREBP1c, Sterol regulatory element-binding protein 1; FASN, fatty acid synthase; ACLY, ATP citrate lyase.
|
 | Fig. 3Body weight, daily food intake, and food efficiency ratio analysis in mice fed on a Western diet (WD) supplemented in the presence or absence of 1% ethanol extract of Allium fistulosum (EAF) for 12 weeks.(A) WD-fed mice exhibit increased body weight compared to the controls, while 1% EAF supplementation significantly reduced body weight. Data are expressed as mean ± SE (n = 7/group). (B) The average weight gain after 12-week supplementation is expressed as mean ± SE (n = 7/group). (C) Daily food intake (g) in the WD-fed group is lower than in their control counterparts, but there are no significant differences among the three groups. The data are expressed as mean ± SE (n = 7/group). (D) The increased food efficiency ratio in WD-fed group was reduced by 1% EAF supplementation. Food efficiency ratio (FER) was calculated by applying the equation: FER = (body weight gain (g)/food intake (g)) × 100. FER is expressed as mean ± SE (n = 7/group). Means not sharing a common superscript are significantly different, P < 0.05.
|
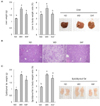 | Fig. 4The 1% ethanol extract of Allium fistulosum (EAF) supplement blocked gains in both liver and epididymal fat weights and attenuated hepatic lipid accumulation.(A) Liver weight was significantly increased in Western diet (WD)-fed mice and was rapidly reduced by 1% EAF supplementation with WD. The data are expressed as mean ± SE (n = 7/group). (B) Mice fed with WD exhibit greater lipid accumulation compared to the controls, while EAF supplementation markedly attenuated this effect. Representative images are shown with a 100 µm scale bar. (C) The epididymal fat weight was significantly increased in WD-fed mice and was reduced by 1% EAF supplementation. The data are expressed as mean ± SE (n = 7/group). Means not sharing a common superscript are significantly different, P < 0.05.
|
 | Fig. 5The 1% ethanol extract of Allium fistulosum (EAF) supplement markedly reduced Western diet (WD)-induced liver abnormalities.(A) Plasma levels of alanine aminotransferase (ALT) were dramatically increased in WD-fed mice but were reduced by 1% EAF supplementation. Values are mean ± SE (n = 7/group). (B) Plasma levels of aspartate transaminase (AST) were statistically increased in WD-fed mice, and 1% EAF supplementation reduced that level; however, there was no statistical significance to the changes. Values are mean ± SE (n = 7/group). (C) The reduced AST/ALT ratio in WD-fed group was dramatically recovered by 1% EAF supplementation. The data are expressed as mean ± SE (n = 7/group). (D) Plasma levels of ALP were significantly increased in WD-fed mice and were reduced by 1% EAF supplementation. Values are mean ± SE (n = 7/group). Means not sharing a common superscript are significantly different, P < 0.05.
|
NAFLD
EAF
IR
TG
FFAs
VLDL
SREBP1c
FASN
ACLY
WD
FER
AST
ALT
ALP
AUTHORS' CONTRIBUTIONS HKC conceived and designed the experiments and also wrote the manuscript. JTH performed the experiments and wrote a draft of the manuscript. MYJ, EJS, and SWJ supported the experiments. JHP critically reviewed the manuscript. HKC supervised the work and critically reviewed the manuscript. All authors read and approved the final manuscript.
Jin-Taek Hwang 
https://orcid.org/0000-0002-6650-7934
Eun Ju Shin 
https://orcid.org/0000-0002-1727-6367
Min-Yu Chung 
https://orcid.org/0000-0001-8981-215X
Jae Ho Park 
https://orcid.org/0000-0003-4428-436X
Sangwon Chung 
https://orcid.org/0000-0001-7773-2195
Hyo-Kyoung Choi 
https://orcid.org/0000-0001-9424-0432