INTRODUCTION
MATERIALS AND METHODS
Materials
Preparation of wheat sprout sample
Extract preparation
Cell cultures and transfection
Cell proliferation assay
Nuclear protein extract
Immunoblot analysis
Immunofluorescence
Statistical analysis
RESULTS
Wheatgrass inhibits the hypoxia-mediated EMT in airway epithelial cells
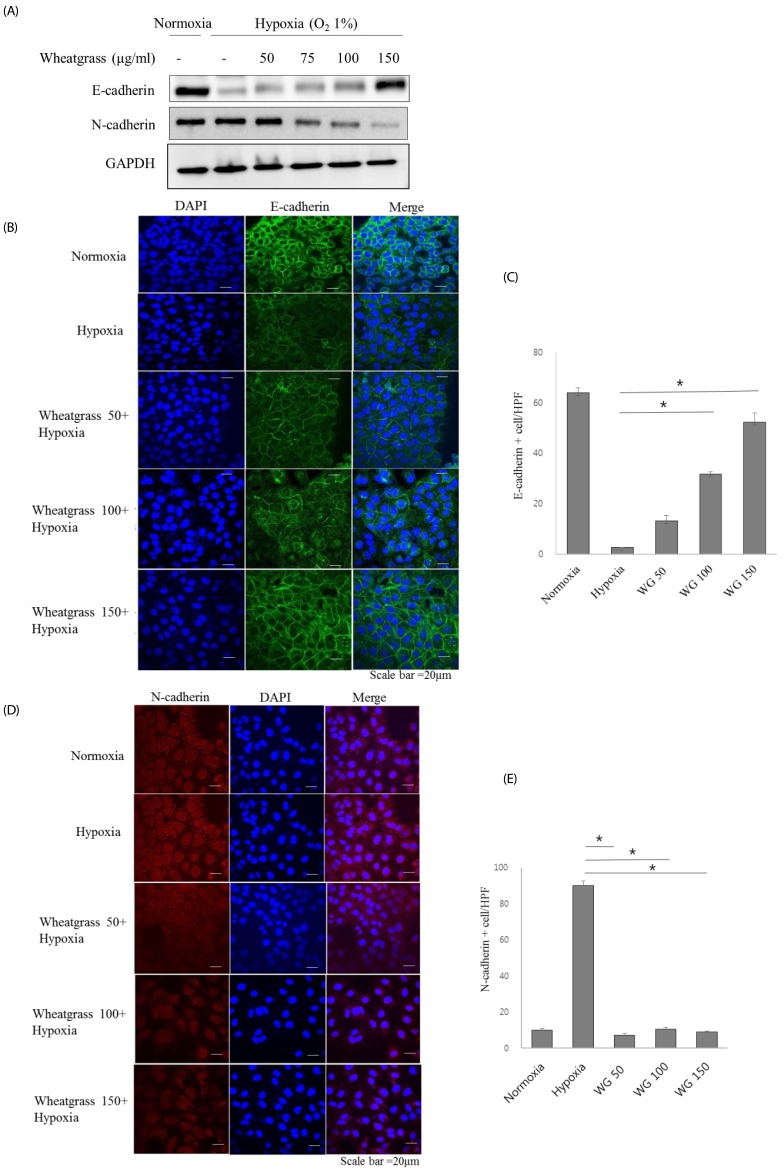 | Fig. 1Wheatgrass inhibits the hypoxia-mediated EMT process in airway epithelial cells.(A) A549 cells were treated with wheatgrass at the indicated concentrations for 24 h and total cell lysates were analyzed by immunoblotting with anti-E-cadherin or N-cadherin antibodies. (B and C) E-cadherin levels were visualized by immunofluorescence analysis. Green fluorescence indicates E-cadherin expression, blue denotes DAPI staining of the nucleus, and the right panel is a merged image of the two panels. Scale bar represents 20 µm. (D and E) N-cadherin expression levels were visualized by immunofluorescence analysis. Red fluorescence indicates E-cadherin expression (*P < 0.01). WG, wheatgrass; EMT, epithelial mesenchymal transition; HPF, high power field.
|
Wheatgrass negatively regulates HIF-1α expression in airway epithelial cells
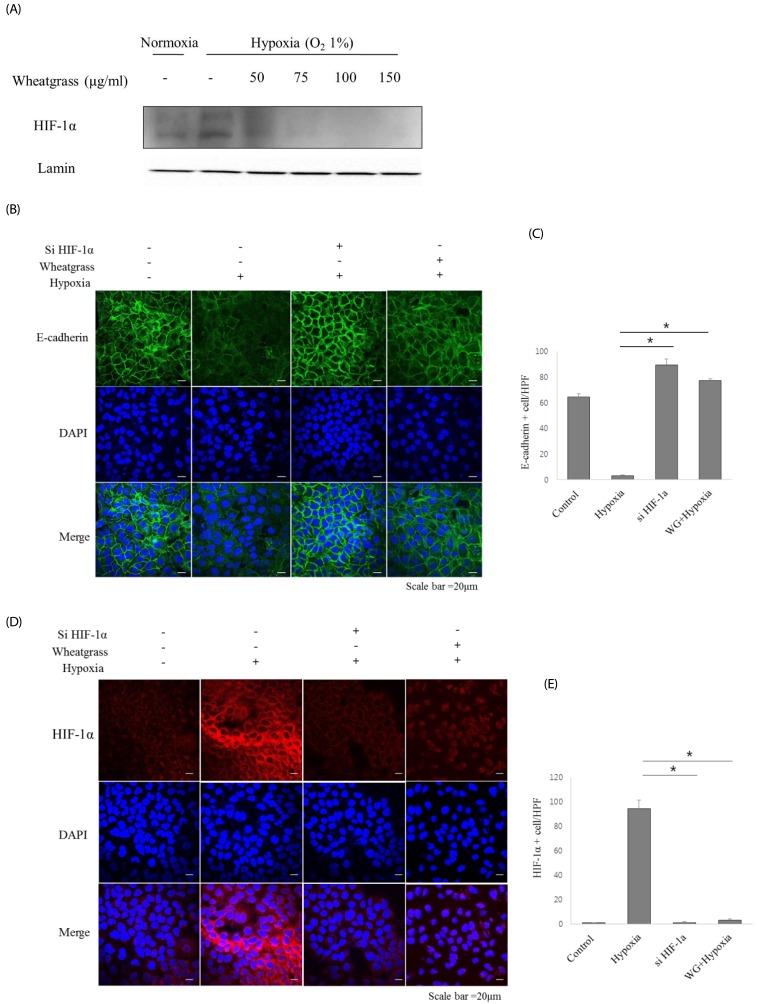 | Fig. 2Wheatgrass inhibits the hypoxia-mediated EMT process by inhibiting the activation of HIF-1α in airway epithelial cells.(A) A549 cells were treated with wheatgrass at the indicated concentrations for 24 h, and nuclear protein fractions were analyzed by immunoblotting with an anti-HIF-1α antibody. (B and C) Cells were transfected with either si-HIF-1α, si-negative control (NC), wheatgrass (150 µg/mL), or hypoxia only as indicated. E-cadherin expression levels (green) were analyzed by immunofluorescence analysis. (D and E) HIF-1α expression levels (red) were visualized by immunofluorescence analysis (*P < 0.01). WG, wheatgrass; EMT, epithelial mesenchymal transition; HIF, hypoxia inducible factor; HPF, high power field.
|
Wheatgrass suppresses EMT by reducing Smad signaling
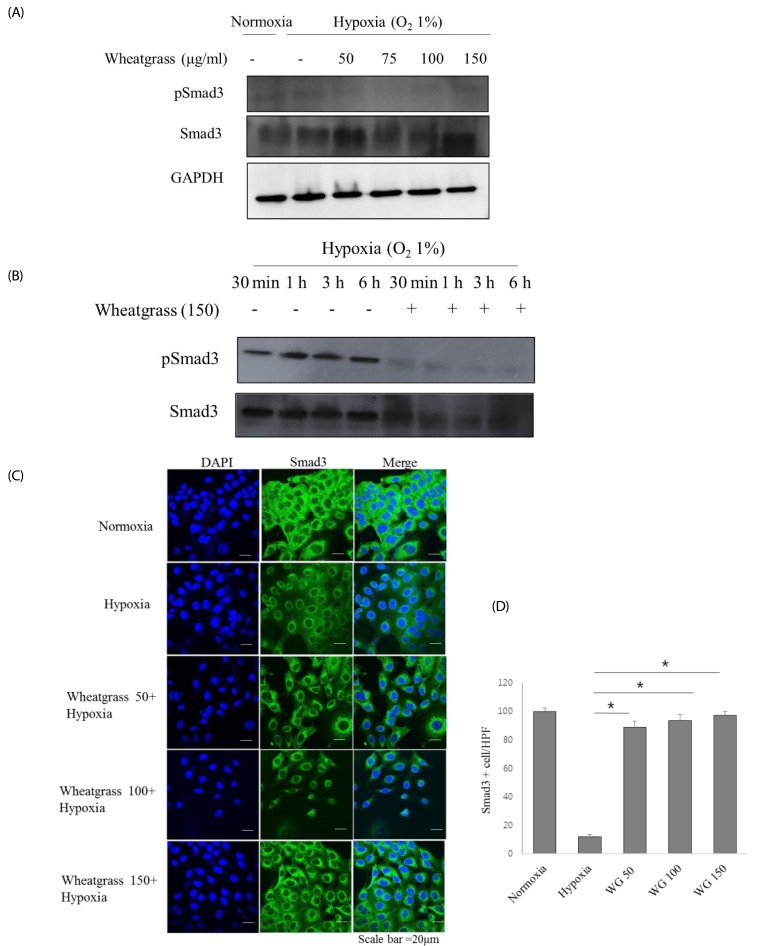 | Fig. 3Wheatgrass inhibits the hypoxia-mediated EMT process by reducing Smad activation.(A and B) Cells were treated and cultured with wheatgrass at the indicated concentrations and times. (C and D) Smad3 expression levels (green) were analyzed by immunofluorescence (*P < 0.01). WG, wheatgrass; EMT, epithelial mesenchymal transition; HPF, high power field.
|
Wheatgrass negatively regulates Snail expression in airway epithelial cells
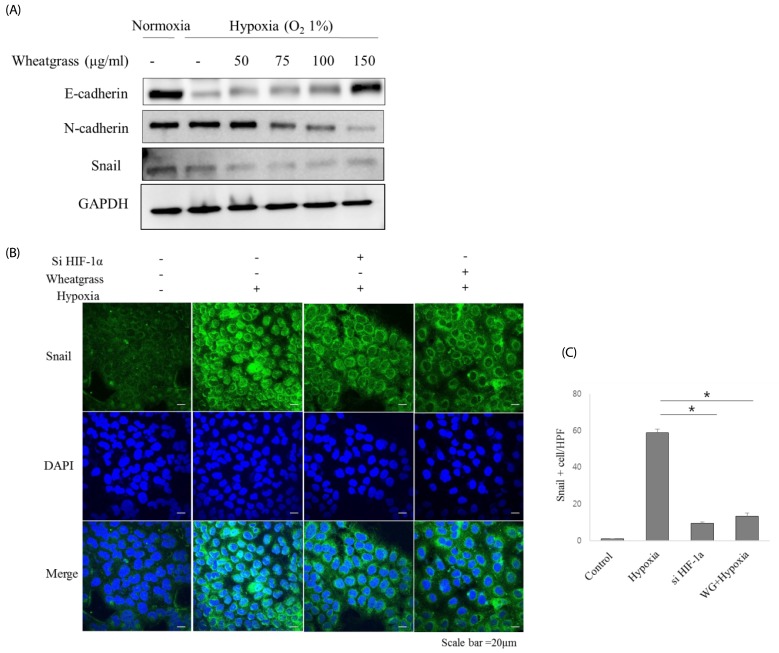 | Fig. 4Wheatgrass negatively regulates Snail expression in airway epithelial cells.(A) A549 cells were treated with wheatgrass at the indicated concentrations for 24 h, and total cell lysates were analyzed by immunoblotting with anti-E-cadherin, N-cadherin, and Snail antibodies. (B and C) Snail expression levels (green) were analyzed by immunofluorescence (*P < 0.01). WG; wheatgrass; HPF, high power field.
|



