Abstract
BACKGROUND/OBJECTIVE
Orostachys japonicus A. Berger (Crassulaceae) has been used in traditional herbal medicines in Korea and other Asian countries to treat various diseases, including liver disorders. In the present study, the anti-fibrotic effects of O. japonicus extract (OJE) in cellular and experimental hepatofibrotic rat models were investigated.
MATERIALS/METHODS
An in vitro hepatic stellate cells (HSCs) system was used to estimate cell viability, cell cycle and apoptosis by MTT assay, flow cytometry, and Annexin V-FITC/PI staining techniques, respectively. In addition, thioacetamide (TAA)-induced liver fibrosis was established in Sprague Dawley rats. Briefly, animals were divided into five groups (n = 8): Control, TAA, OJE 10 (TAA with OJE 10 mg/kg), OJE 100 (TAA with OJE 100 mg/kg) and silymarin (TAA with Silymarin 50 mg/kg). Fibrosis was induced by treatment with TAA (200 mg/kg, i.p.) twice per week for 13 weeks, while OJE and silymarin were administered orally two times per week from week 7 to 13. The fibrotic related gene expression serum biomarkers glutathione and hydroxyproline were estimated by RT-PCR and spectrophotometry, respectively, using commercial kits.
RESULTS
OJE (0.5 and 0.1 mg/mL) and silymarin (0.05 mg/mL) treatment significantly (P < 0.01 and P < 0.001) induced apoptosis (16.95% and 27.48% for OJE and 25.87% for silymarin, respectively) in HSC-T6 cells when compared with the control group (9.09%). Further, rat primary HSCs showed changes in morphology in response to OJE 0.1 mg/mL treatment. In in vivo studies, OJE (10 and 100 mg/kg) treatment significantly ameliorated TAA-induced alterations in levels of serum biomarkers, fibrotic related gene expression, glutathione, and hydroxyproline (P < 0.05-P < 0.001) and rescued the histopathological changes.
Liver fibrosis is a chronic disease state that typically results from dysfunctional wound healing in response to tissue injury [1]. Historically, liver fibrosis is believed to be a passive and irreversible process because of the collapse of the hepatic parenchyma and its substitution with a collagen-rich tissue [2]. Liver fibrosis, which is the common response of the liver to toxins, viral infections, drugs and various metabolic agents, is characterized by excessive accumulation of extracellular matrix (ECM) ultimately leading to cirrhosis and cancer [3]. Overproduction and irregular deposition of ECM in liver tissues leads to the distortion of hepatic microstructure and liver dysfunction. Thus, fibrotic stage is very important in the progress of liver disease [4].
Hepatic stellate cells (HSCs), the key cell type involved in liver fibrosis, normally exist in a quiescent state. Upon activation, HSCs undergo phenotypic changes that lead to secretion of an ECM scar for protection against liver damage. However, if the liver does not recover, this proceeds to liver fibrosis, cirrhosis and cancer. Therefore, it is important to induce the apoptosis of HSCs or prevent the secretion of the ECM by HSCs [56]. It is well known that the thioacetamide (TAA)-induced hepatic fibrosis experimental model resembles human liver fibrosis with respect to hemodynamics, morphology and biochemical metabolism, that it is also similar to virus-induced cirrhosis [7]. Many chronic liver diseases share the pathological process of hepatic fibrosis.
Orostachys japonicus (O. japonicus) A. Berger (Crassulaceae) is a traditional medicinal herb that grows in the barren soil of mountains or roof tiles. In traditional Asian medicine, O. japonica is used to reduce swelling, pain, and bleeding, as well as in the treatment of hematemesis, epistaxis, acute infectious type non-jaundice, hepatitis, malaria, bladder stones, gonorrhea, eczema and pneumonia [89]. Pharmacologically, O. japonicus has been reported to possess anti-oxidant [10], anti-microbial [11], hypolipidemic and hypoglycemic effects [12]. However, there is currently no scientific evidence of the anti-hepatofibrotic effects of O. japonica. Thus, in the present study, we investigated the anti-fibrotic effects of O. japonica extracts in an in vitro system using HSCs and in vivo using a TAA-induced hepatic fibrosis rat model.
Silymarin, hydroxyproline, p-dimethylaminobenzaldehyde,1,1, 3,3-tetraethoxypropane (TEP), chloramines-T, 5,5-dithiobis-2-nitrobenzoic acid (DTNB), glutathione (GSH), β-nicotinamide adenine dinucleotide phosphate, reduced form (β-NADPH), TAA and other reagents were purchased from Sigma, St. Louis, MO, USA. Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were acquired from Invitrogen (Carlsbad, CA, USA). Perchloric acid was obtained from GFS Chemical Co. (Columbus, OH, USA). A GOT-GPT assay kit was purchased from Asan Pharmaceutical (Hwaseong-si, Korea). Annexin V-FITC and PI Apoptosis Detection Kit I were acquired from BD Biosciences (San Jose, CA, USA). All other reagents used in this study were of the highest grade available commercially.
The plant material of O. japonica collected from June-July, 2016, was purchased from Chinese Medicinal Plant Co., Jecheon, South Korea. For extraction, dried O. japonica (100 g) was ground to a fine powder and extracted with 1 L ethanol (95%) using Soxhlet's extraction technique for three days. The extract was then concentrated in a vacuum under reduced pressure and lyophilized. The final yield of the lyophilized O. japonica extract (OJE) was 22.5% (w/w). The lyophilized OJE was stored at 4℃ until use. Prior to use, the lyophilized OJE was dissolved in 10% dimethyl sulfoxide (DMSO; Junsei Chemical Co., Ltd., Tokyo, Japan) and filtered through a 0.22 µM syringe filter, after which it was stored as a stock until use in each experiment. The final concentration of DMSO used for the study was not more than 0.1%.
The immortalized rat Hepatic Stellate Cell lines (HSC-T6) were received from Prof. Chang-Gue Son (Korean Hospital of Daejeon University, Daejeon, Korea). The Chang liver cell line was purchased from the American Type Culture Collection (Manassas, VA, USA). HSC-T6 cells were cultured in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 5% FBS and 1% antibiotic-antimycotic (Invitrogen) in a humidified atmosphere of 5% CO2 at 37℃. The Chang liver cell line was used as a normal human cell line derived from normal liver tissue. The cells were cultured in DMEM supplemented with 10% FBS (Invitrogen, Carlsbad, CA, USA) and 1% antibiotic-antimycotic in a humidified atmosphere of 5% CO2 at 37℃. For activation, HSCT6 cells were serum starved before treatment with OJE
Hepatic stellate cells were isolated from 7-week-old male Sprague Dawley (SD) rats by in situ pronase, collagenase perfusion, and single-step Histogenz gradient as previously described [1314]. Isolated HSCs were cultured in DMEM containing 10% FBS and 1% antibiotic-antimycotic maintained in a humidified atmosphere of 5% CO2 at 37℃. Growth medium was changed on a daily basis for 7 days.
Cell viability in HSC-T6 cells was measured using the 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT; Sigma, St. Louis, MO, USA) method. HSC-T6 (6 × 105 cells/well) were cultured in a 96-well plate with DMEM containing 10% FBS and 1% antibiotic-antimycotic (Invitrogen, Carlsbad, CA, USA) maintained in a humidified atmosphere of 5% CO2 at 37℃. Next, HSC-T6 cells were treated with various concentrations of OJE (0, 0.01, 0.1, 0.5, 1.0 mg/mL) for 24 h. Cells were then incubated with 0.5 mg/mL MTT for 3 h, after which the reaction was interrupted by DMSO. The extent of reduction of MTT to formazan was measured using an ELISA reader at an optical density of 540 nm based on comparison to the viabilities of the control cells.
HSC-T6 cells (1.5 × 106 cells/well) were cultured in DMEM containing 10% FBS and 1% antibiotic-antimycotic maintained in a humidified atmosphere of 5% CO2 at 37℃. Growth medium was changed on a daily basis for 7 days. For cell cycle analysis, sample materials of OJE 0.1 and 0.5 mg/mL were evaluated for 24 h at 37℃ in an atmosphere of 5% CO2 and 95% humidity. Cells were then washed with PBS twice, labelled with 1 mL cold propidium iodide (PI) solution (50 µg/mL PI and 100 µg/mL RNase A) and incubated on ice for 30 min in the dark. Data were analyzed by flow cytometry (FACSCalibur, BD Biosciences, San Jose, CA, USA).
HSC-T6 cells were treated with OJE (0.1 and 0.5 mg/mL) and silymarin (0.05 mg/mL) for 24 h and harvested for apoptosis assay by using Annexin V-FITC and PI Apoptosis Detection Kit I (BD Biosciences, San Jose, CA, USA) according to the manufacturer's instructions. Data were analyzed using the BD CellQuest software (BD Biosciences, San Jose, CA, USA), which allowed assessment of specific populations. Individualization by gates was conducted according to size (FSC), granularity (SSC), and fluorescent (FL) parameters. Both early apoptotic (Annexin V+ and PI-) and late apoptotic (Annexin V+ and PI+) cells were included in cell death determinations.
Total RNA was extracted from liver tissue samples and HSC-T6 cells using TRIzol reagent (Qiagen, Valencia, CA, USA). cDNA was synthesized from total RNA (2 µg) in a 20 µL reaction using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster, CA, USA). The primers for α-smooth muscle actin (α-SMA), collagen type1 alpha 1 (Col1α1), transforming growth factor β1 (TGF-β1), and β-actin were as follows. α-SMA (forward sequence, 5′-AACACGGCATCATCACCAACT-3′; reverse sequence, 5′-TTTCTCCCGGTTGGCCTTA-3′, Col1α1 (forward sequence, 5′-CCCAGCGGTGGTTATGACTT-3′; reverse sequence, 5′-GCTGCGG ATGTTCTCAATCTG-3′), TGF-β1 (forward sequence, 5′-AGGAGA CGGAATACAGGGCTTT-3′; reverse sequence, 5′-AGC AGGAAGGG TCGGTTCAT-3′), β-actin (forward sequence, 5′-CTAAGGCCAACC GTGAAAAGAT-3′; reverse sequence, 5′-GACCAGAGGCATACAGG GACAA-3′). The processes of reactions were conducted according to the manufacturer's instructions. For analysis of data, the gene expression levels were compared with those of β-actin as a reference gene.
Six week old specific-pathogen-free male SD rats (six-weeks old, 190-210 g) were purchased from a commercial animal breeder (Orient Bio, Seongnam, Korea). The animals were housed in conventional cages under controlled temperature (23 ± 3℃), relative humidity (50 ± 20%) and a 12 h light/dark cycle with free access to food and water. Animals were allowed to acclimatize for at least one week before use. All animal experiments were approved by the Committee of Laboratory Animals according to the Institutional Guidelines of Konkuk University, Republic of Korea (IACUC No. KU15017).
Rats were randomly divided into the following five groups of eight animals each: control group (injected with normal saline intra-peritoneally (i.p)), TAA group, OJE 10 (TAA with OJE 10 mg/kg), OJE 50 (TAA with OJE 50 mg/kg), and positive control silymarin group (TAA with 50 mg/kg silymarin). TAA (200 mg/kg) was injected (i.p) twice a week for 13 weeks to four groups except control group. OJE (10 or 50 mg/kg), silymarin (50 mg/kg), or vehicle (sterile distilled water) was administered by gastric gavage two times per week from week 7 to 13. After the last treatments, animals were fasted for 18 h, after which blood was collected by cardiac puncture under CO2 anesthesia. Liver tissues were removed and stored at -80℃ until use for measurement of the expression of hydroxyproline, GSH, and fibrosis-related mediated genes. A portion of the liver tissue was fixed in Bouin's solution for histo-morphological findings and another small portion was fixed in RNAlater solution for gene expression studies.
On the final day of the experiment, blood samples were collected into heparinized tubes via cardiac puncture under CO2 anesthesia. Blood was immediately processed by centrifugation at 3,500 g for 15 min. Next, serum levels of aspartate transaminase (AST) and alanine transaminase (ALT) were measured by spectrophotometry using a commercially available GOT-GPT assay kit (Asan Pharmaceutical, Hwaseong-si, Korea).
We measured the levels of GSH using a spectrophotometer according to Ellman's method [15]. Briefly, a 50 µL sample of homogenate (or GSH standard) was combined with 80 µL of freshly prepared DTNB/NADPH mixture (10 µL 4 mM DTNB and 70 µL 0.3 mM NADPH) in a 96-well plate. Finally, 20 µL (0.06 U) of GSH reductase solution was added to each well and the absorbance was recorded at 412 nm after 5 min. The amount of GSH was expressed as mM of GSH per gram of tissue.
Hydroxyproline determination was conducted as previously described, with some modifications [16]. Briefly, liver tissues were homogenized with dilution buffer. Hydrolysis was performed by adding 1 mL of 6N HCl to 2 mL of liver homogenate in a tightly capped glass tube and then incubating samples overnight at 100℃. After cooling, the acid hydrolysates were filtered through a 0.45 µm filter paper (Toyo Roshi Kaisha, Tokyo, Japan). Next, 50 µL samples or hydroxyproline standards in 6N HCl were air-dried and dissolved in methanol (50 µL), after which 1.2 mL of 50% isopropanol and 200 µL of chloramine-T solution were added to each sample, which was followed by incubation at room temperature for 10 min. Ehrlich's solution (1.3 mL) was then added, after which the samples were incubated at 50℃ for 90 min and the optical density of the reaction product was read at 558 nm using a spectrophotometer (Sunrise, Tecan, San Jose, CA USA). Concentrations were then determined based on comparison to a standard curve constructed using serial dilutions of 0.5 mg/mL hydroxyproline solution.
Liver tissues were fixed in Bouin's solution and then embedded in paraffin, after which paraffin sections of 5 µm thickness were stained with hematoxylin and eosin (H & E) and Masson's trichrome. For identification and analysis of collagen expression, the blue-stained areas in the Masson's trichrome stained sections were measured using an image analyzer (Image J, NIH, Bethesda, MD, USA).
We first assessed the effect of various concentrations (0, 0.01, 0.1, 0.5 and 1.0 mg/mL) of OJE on cell viability in HSC-T6 cells and Chang liver cells. As shown in Fig. 1A, OJE administered at concentrations of up to 0.5 mg/mL did not show induce any significant changes in the overall cell viability or generate toxicity in HSC-T6 cells or Chang liver cells. However, 1.0 mg/mL induced significant changes, negatively affecting the overall cell viability. Furthermore, the solvent used to dissolve the OJE extract, DMSO (0.1%), also did not show any toxicity toward HSC-T6 and Chang liver cells. Therefore, all in vitro experiments were conducted using OJE 0.1 and/or 0.5 mg/mL as the concentrations were considered non-toxic and effective (Fig. 1A).
As shown in Fig. 1B, untreated activated HSCs showed normal morphology (day 7). Moreover, treatment of 8 day-cultured primary HSCs with OJE at 0.5 mg/mL (high non-toxic concentration) for 24 h reduced collagen fiber morphology and decreased the number of viable HSCs. Further, decreased stretched fibers were observed after 24 h when compared to untreated activated HSCs. Taken together, these findings clearly indicate that OJE influenced the morphology of the cultured activated HSCs (Fig. 1C).
As shown in Fig. 2, flow cytometric analysis of OJE treated HSC-T6 cells showed marked effects. Specifically, treatment with 0.1 mg/mL and 0.5 mg/mL OJE resulted in 1.79% and 2.55% of the cells compared with control cells showing a distribution of 1.08% in the sub-G1 phase. Additionally, treatment with silymarin resulted in 1.65% of the cells being in the sub-G1 phase. To verify the apoptosis effect of OJE, HSC-T6 cells were treated with silymarin (0.05 mg/mL) and OJE (0.1 and 0.5 mg/mL) for 24 h. As indicated by the Annexin V-FITC/PI assay, OJE and silymarin significantly increased apoptosis in HSC-T6 cells when compared with the control group (Fig. 3A-3D). The percentage of cells undergoing apoptotic cell death increased from 9.09 ± 1.54 to 25.87 ± 3.65 in the silymarin group, while it was 27.48 ± 1.89 in the 0.5 mg/mL OJE treated group and 16.95 ± 3.01% in the 0.1 mg/mL OJE treated group at 24 h. When compared with the Annexin V positive cells in the control group, OJE (0.5 mg/mL) and silymarin (0.05 mg/mL) induced three and two fold increases in Annexin V positive cells, respectively (Fig. 3E). Taken together, these results show that OJE significantly (P < 0.01) induced cell death and apoptosis in HSC-T6 cells.
The expression of genes associated with fibrosis such as TGF-β, α-SMA and Col1α1 were measured to verify the effects of OJE on HSC-T6 cells. As shown in Fig. 3F, TGF-β, α-SMA and Col1α1 expression were significantly down-regulated in the OJE (0.1 and 0.5 mg/mL) treatment groups when compared with the control group. The positive control silymarin also exhibited significant effects by regulating the fibrosis mediated gene suppression that were similar to the effects of the OJE 0.5 mg/mL treated group.
TAA treatment significantly elevated the serum ALT and AST levels when compared with the control group (Fig. 4A and 4B). The elevated levels of ALT and AST were significantly attenuated by OJE at both concentrations (10 and 50 mg/kg) when compared with TAA-induced group. Silymarin also exhibited a significant effect when compared with control treated groups.
As shown in Fig. 4C, TAA treatment significantly decreased (P < 0.001) the GSH contents in the liver tissue when compared with the control group. However, OJE (10 and 50 mg/kg) treatment significantly (P < 0.01) attenuated this decrease in GSH contents, as did treatment with the positive control silymarin (P < 0.001). These findings indicate that TAA causes liver injury through reactive oxygen species (ROS) damage, which is associated with antioxidant enzymes, and that OJE significantly ameliorated these changes.
The hydroxyproline content in TAA-induced liver tissue was significantly (P < 0.001) increased in the TAA group compared to the normal group. However, OJE (10 and 50 mg/kg) treatment significantly reduced hydroxyproline levels compared with the TAA group (Fig. 4D). The silymarin treatment also reduced hydroxyproline content in TAA-induced liver tissue.
As shown in Fig. 5A, the control group showed normal morphology upon H & E staining. However, TAA treatment led to severe pathological alterations, such as a shrunken, solidified and abnormally patterned liver (Fig. 5B). These alterations were dramatically attenuated by OJE (10 and 50 mg/kg) and silymarin treatment (Fig. 5C-5E). Further, Masson's trichrome showed severe collagen accumulation (part of the blue staining) in the TAA group, while the OJE (10 and 50 mg/kg) treated groups remarkably protected against collagen accumulation (Fig. 6A-6E). The percentage area of fibrosis revealed significant damage in the TAA treated group when compared with the control group. However, treatment with OJE at both concentrations and silymarin ameliorated these changes significantly (P < 0.001 and P < 0.01, respectively) when compared with TAA treated group (Fig. 6F).
Fibrosis associated genes expression in the liver tissue was analyzed using qRT-PCR. TAA treatment up-regulated the gene expression of TGF-β, α-SMA and Col1α1, while OJE (50 mg/kg) and positive control silymarin treated groups down-regulated the expression of all genes tested (Fig. 7A-7C). However, OJE treatment at 10 mg/kg did not influence the TAA-induced changes in α-SMA expression (Fig. 7B). Further, treatment with silymarin exhibited superior effects at down regulating the expression of fibrotic associated genes than OJE, which was in agreement with the in vitro data.
The liver is an organ of paramount importance that plays a major role in regulating various physiological processes including metabolism, excretion and regulation of glycogen storage in the body. Fibrosis of the liver is a reversible complication of advanced hepatic disease that represents a major burden in health care. Hepatic stellate cells (HSCs), which are the major cell type involved in the production of ECM in the liver, undergo activation into proliferative and fibrogenic myofibroblast-like cells during liver injury [7]. Dysregulation in the proliferation and apoptosis of HSC is known to be involved in the pathogenesis of liver fibrosis; therefore, inhibiting HSC activation and inducing apoptosis has become an ideal approach to preventing or treating hepatic fibrosis [71718]. In the present study, OJE inhibited HSC proliferation, induced apoptosis and altered the morphology of HSCs in vitro. Further, OJE (0.5 mg/mL) down regulated the gene expression of TGF-β, α-SMA, and Col1α1, selective markers of HSCs activation.
In in vivo studies, liver fibrosis is commonly induced with thioacetamide, which is readily metabolized to reactive acetamide and TAA-S-oxide. These metabolites react with hepatic tissue, leading to the accumulation of fatty acids, DNA/protein damage and formation of reactive oxygen species (ROS). The major changes observed in TAA-induced liver damage are altered serum liver enzymes such as AST and ALT [1920]. In this study, the TTA-induced increase in the levels of AST and ALT enzymes was significantly reduced by OJE treatment. Furthermore, the ROS generated by the metabolites of TAA led to progression of liver damage and reduced the levels of antioxidant enzymes such as GSH [21]. Therefore, restoring GSH content might be helpful to the treatment of TAA-induced oxidative liver damage. Accumulation of ECM is a common phenomenon in liver fibrosis, and hydroxyproline, a major constituent of collagen, is a good marker of ECM accumulation [2223]. In the present study, OJE markedly restored the GSH content and decreased the hydroxyproline level, indicating that this compound inhibited ROS generation and ECM accumulation, thereby suppressing hepatic fibrosis.
Earlier reports indicated that inhibition of the TGF-β1 signaling pathway attenuates liver fibrosis. Signaling of TGF-β family members is mediated by TGFβR, which phosphorylates downstream receptor-activated Smads, which are considered specific markers for smooth muscle cell differentiation [2425]. Further, TGF-β produced by Kupffer cells and HSCs, up regulates transcription of the collagen genes Col1α1 and Col1α2, which are observed in damaged liver and highly expressed in activated HSCs from cirrhotic liver [26]. Therefore, we tested the effects of OJE on the expression of genes involved in fibrotic mediation such as of TGF-β, Col1α1 and α-SMA in rat liver tissues induced by TAA. In agreement with our in vitro data, OJE significantly suppressed the TAA-induced increase in the expression of TGF-β, Col1α1 and α-SMA in rat liver tissues. These results indicate that the antifibrotic action of OJE might be partially mediated via inhibition of the TGF-β1/Smad pathway. Further, histopathological studies revealed that TAA intoxication produced substantial liver fibrosis and prominent regenerative nodule development, resulting in features of degeneration, necrosis and elements of fibrosis [27]. These alterations in TAA-induced morphological features were attenuated by OJE.
O. japonicus is an important health promoting herb with rich nutritional values [12]. Reports have indicated the presence of numerous bioactive constituents including dietary flavonoids (quercetin and kaempferol), fatty acid esters, triterpenoids, 4-hydroxybenzoic acid, 3,4-dihydroxybenzoic acid, and gallic acid [282930]. Some of these compounds, such as quercetin, kaempferol and gallic acid, were reported to possess strong hepatoprotective properties including anti-fibrotic effects [313233]. The compounds present in the OJE might act synergistically in delivery of potent anti-hepatofibrotic effects.
In conclusion, OJE exhibited potential anti-fibrotic effects by increasing the apoptosis and inhibition of ECM accumulation in HSC-T6 cells. Furthermore, OJE ameliorated the TAA-induced liver fibrosis in vivo in a rat model. Regulation of the TGF-β 1/Smad pathway and anti-oxidative potential might partly be the mechanistic basis for the potent effects shown by OJE as an anti-hepatoprotective agent. The results of the present study provide scientific evidence for the traditional claims of the usefulness of OJE to treat liver disorders. Based on these observations, we suggest that further studies be conducted to investigate development of O. japonicus as a potential nutritional supplement against toxin and oxidative stress mediated liver fibrosis.
Figures and Tables
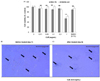 | Fig. 1Cell viability assay of HSC-T6/Chang liver cells and morphological changes in primary HSCs in response to treatment with OJE.(A) HSC-T6 and Chang liver cells were incubated with OJE at the indicated concentrations for 24 h, after which the cell viability was determined by MTT assay. Primary HSCs were cultivated for 1 week (B) and exposed to OJE at 0.5 mg/mL for 24 h (C). Pictures were taken before and after 24 h of treatment with OJE. Magnification was 100×. Arrows indicate HSCs. The data are expressed as the means ± SEM (n = 8), which were compared using one-way analysis of variance (ANOVA) followed by Student's t-test. NS, not significant and ***P < 0.001 compared to the control group. OJE, O. japonica extract; HSC, hepatic stellate cells; DMSO, dimethyl sulfoxide.
|
 | Fig. 2Effect of OJE on the cell cycle in HSC-T6 cells.DNA contents in different phases of the cell cycle were measured by flow cytometry using propidium iodide. The cell cycle distribution for each treatment group and the percentage of the cell cycle distribution are represented by graphs (A) and histogram (B), respectively. OJE: O. japonica extract.
|
 | Fig. 3Effect of OJE on apoptosis and fibrosis expression in HSC-T6 cells.A: Control cells. Flow cytometric data indicate apoptosis in HSC-T6 cells after incubation with silymarin (B), OJE 0.5 mg/mL (C), or OJE 0.1 mg/mL (D) for 24 h. E: Data show the apoptotic (Annexin V+ and PI-) and late apoptotic (Annexin V+ and PI+) cells. F: Fibrosis related gene expression. The data are presented as the means ± SEM (n = 8) which were compared using one-way analysis of variance (ANOVA) followed by Student's t-test. * P < 0.05 and ** P < 0.01 compared to the control group. OJE: O. japonica extract.
|
 | Fig. 4Effect of OJE on AST/ALT levels, total glutathione (GSH) contents and hydroxyproline levels in TAA-induced liver fibrosis rats.A: HSC-T6 cells were incubated with 10 and 50 mg/mL OJE for 24 h. Expression of fibrosis related genes in HSC-T6 cells was determined by real-time PCR. The results are expressed as normalized fold values relative to the control. Levels of ALT (A) and AST (B) in serum were measured by spectrophotometry. (C) Total GSH contents in liver tissue (D) and hydroxyproline levels of TAA-induced liver tissue of rats were measured using spectrophotometry. TAA, thioacetamide-induced liver fibrosis rats; Silymarin, positive control rats; OJE 10, TAA plus OJE 10 mg/kg treated rats; OJE 50, TAA plus OJE 50 mg/kg treated rats. Data are expressed as means ± SEM (n = 8) which were compared using one-way analysis of variance (ANOVA) followed by Student's t-test. ###P < 0.001 compared to the control group, *P < 0.05, **P < 0.01, ***P < 0.001 compared with TAA group. OJE, O. japonica extract; TAA, thioacetamide; ALT, alanine transaminase; AST, aspartate transaminase; GSH, glutathione.
|
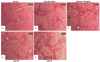 | Fig. 5Effect of OJE on TAA-induced morphology of liver tissues by Hematoxylin and Eosin (H&E) staining.At the end of the experiment, all animals were sacrificed and livers were fixed in Bouin's solution. After staining with H&E, liver sections were observed by light microscopy. Control, naive rats (A); TAA, TAA-induced liver fibrosis rats (B); Silymarin, Positive control rats (C); OJE 50, TAA plus OJE 50 mg/kg treated rats (D); OJE 10, TAA plus OJE 10 mg/kg treated rats (E). Scale bar = 200 µm. OJE, O. japonica extract; TAA, thioacetamide.
|
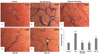 | Fig. 6Effect of OJE on TAA-induced fibrosis by Masson's trichrome staining of liver tissues.This stain was performed as described for H&E staining. Control, naive rats (A); TAA, TAA-induced liver fibrosis rats (B); Silymarin, Positive control rats (C); OJE 50, TAA plus OJE 50 mg/kg treated rats (D); OJE 10, TAA plus OJE 10 mg/kg treated rats. Percentage of fibrosis area plot (F). Scale bar = 200 µm. Quantification was accomplished using ImageJ. Values are presented as the means ± SEM (n = 8) which were compared using one-way analysis of variance (ANOVA) followed by Student's t-test. #P < 0.05 compared to the control group, **P < 0.01, ***P < 0.001 compared to the TAA group. TAA, Thioacetamide; OJE, O. japonica extract.
|
 | Fig. 7Effect of OJE on fibrosis related gene expression in TAA-induced liver tissues.Expression of fibrosis related genes in liver tissue was measured by real-time PCR. (A) TGF-β, (B) α-SMA, (C) Col1α1. The results are expressed as normalized fold values relative to the control. Values are presented as the means ± SEM (n = 8) which were compared using one-way analysis of variance (ANOVA) followed by Student's t-test. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the TAA group. TAA, Thioacetamide; OJE, O. japonica extract; α-SMA, alpha-smooth muscle actin; Col1α1, collagen type1 alpha 1; TGF-β1, transforming growth factor β1.
|
References
1. Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014; 7:193–203.

2. Popper H, Uenfriend S. Hepatic fibrosis. Correlation of biochemical and morphologic investigations. Am J Med. 1970; 49:707–721.
3. Friedman SL, Maher JJ, Bissell DM. Mechanisms and therapy of hepatic fibrosis: report of the AASLD Single Topic Basic Research Conference. Hepatology. 2000; 32:1403–1408.

4. Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000; 287:1253–1258.

5. Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001; 21:311–335.

6. Lee JJ, Yang SY, Kim DH, Hur SJ, Lee JD, Yum MJ, Song MD. Liver fibrosis protective effect of Hovenia dulcis fruit. Curr Top Nutraceutical Res. 2014; 12:43–50.
7. Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008; 88:125–172.

8. Lee SJ, Shin JH, Kang JR, Hwang CR, Sung NJ. In vitro evaluation of biological activities of Wa-song (Orostachys japonicus A. Berger) and Korean traditional plants mixture. J Korean Soc Food Sci Nutr. 2012; 41:295–301.

9. Kim JK. Illustrated Natural Drugs. Seoul: Namsandang Publishing Co;1984.
10. Lee SJ, Seo JK, Shin JH, Lee HJ, Sung NJ. Antioxidant activity of Wa-song (Orostachys japonicus A. Berger) according to drying methods. J Korean Soc Food Sci Nutr. 2008; 37:605–611.

11. Yoon JA, Son YS. Effects of Opuntia ficus-indica complexes B (OCB) on blood glucose and lipid metabolism in streptozotocin-induced diabetic rats. Korean J Food Nutr. 2009; 22:48–56.
12. Lee SJ, Zhang GF, Sung NJ. Hypolipidemic and hypoglycemic effects of Orostachys japonicus A. Berger extracts in streptozotocin-induced diabetic rats. Nutr Res Pract. 2011; 5:301–307.

13. Hendriks HF, Verhoofstad WA, Brouwer A, de Leeuw AM, Knook DL. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res. 1985; 160:138–149.

14. Knook DL, Seffelaar AM, de Leeuw AM. Fat-storing cells of the rat liver. Their isolation and purification. Exp Cell Res. 1982; 139:468–471.

16. Takayama T, Fujita K, Suzuki K, Sakaguchi M, Fujie M, Nagai E, Watanabe S, Ichiyama A, Ogawa Y. Control of oxalate formation from L-hydroxyproline in liver mitochondria. J Am Soc Nephrol. 2003; 14:939–946.

17. Gressner AM. The cell biology of liver fibrogenesis - an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998; 292:447–452.

18. Friedman SL. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004; 1:98–105.

19. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999; 59:2223–2230.
20. McClatchey KD. Clinical Laboratory Medicine. 2nd ed. Philadelphia (PA): Lippincott Wiliams & Wilkins;2002.
21. Bruck R, Aeed H, Avni Y, Shirin H, Matas Z, Shahmurov M, Avinoach I, Zozulya G, Weizman N, Hochman A. Melatonin inhibits nuclear factor kappa B activation and oxidative stress and protects against thioacetamide induced liver damage in rats. J Hepatol. 2004; 40:86–93.

22. Krane SM. The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino Acids. 2008; 35:703–710.

23. Pálfi VK, Perczel A. How stable is a collagen triple helix? An ab initio study on various collagen and beta-sheet forming sequences. J Comput Chem. 2008; 29:1374–1386.

24. Verrecchia F, Mauviel A. Transforming growth factor-beta and fibrosis. World J Gastroenterol. 2007; 13:3056–3062.
25. Liu Y, Wang Z, Kwong SQ, Lui EL, Friedman SL, Li FR, Lam RW, Zhang GC, Zhang H, Ye T. Inhibition of PDGF, TGF-beta, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011; 55:612–625.

26. Jakowlew SB, Mead JE, Danielpour D, Wu J, Roberts AB, Fausto N. Transforming growth factor-beta (TGF-beta) isoforms in rat liver regeneration: messenger RNA expression and activation of latent TGF-beta. Cell Regul. 1991; 2:535–548.

27. Hori N, Okanoue T, Sawa Y, Mori T, Kashima K. Hemodynamic characterization in experimental liver cirrhosis induced by thioacetamide administration. Dig Dis Sci. 1993; 38:2195–2202.

28. Park HJ, Young HS, Park KY, Rhee SH, Chung HY, Choi JS. Flavonoids from the whole plants of Orostachys japonicus. Arch Pharm Res. 1991; 14:167–171.
29. Yoon Y, Kim KS, Hong SG, Kang BJ, Lee MY, Cho DW. Protective effects of Orostachys japonicus A. Berger (Crassulaceae) on H2O2-induced apoptosis in GT1-1 mouse hypothalamic neuronal cell line. J Ethnopharmacol. 2000; 69:73–78.

30. Yoon NY, Min BS, Lee HK, Park JC, Choi JS. A potent anti-complementary acylated sterol glucoside from Orostachys japonicus. Arch Pharm Res. 2005; 28:892–896.

31. Hernández-Ortega LD, Alcántar-Díaz BE, Ruiz-Corro LA, Sandoval-Rodriguez A, Bueno-Topete M, Armendariz-Borunda J, Salazar-Montes AM. Quercetin improves hepatic fibrosis reducing hepatic stellate cells and regulating pro-fibrogenic/anti-fibrogenic molecules balance. J Gastroenterol Hepatol. 2012; 27:1865–1872.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download