Abstract
BACKGROUND/OBSECTIVE
Airway inflammation by eosinophils, neutrophils and alveolar macrophages is a characteristic feature of asthma that leads to pathological subepithelial thickening and remodeling. Our previous study showed that oxidative stress in airways resulted in eosinophilia and epithelial apoptosis. The current study investigated whether glutathione-containing dry yeast extract (dry-YE) ameliorated eosinophilia, goblet cell hyperplasia and mucus overproduction.
MATERIALS/METHOD
This study employed 2 µg/mL lipopolysaccharide (LPS)- or 20 ng/mL eotaxin-1-exposed human bronchial epithelial cells and ovalbumin (OVA)-challenged mice. Dry-YE employed in this study contained a significant amount of glutathione (140 mg in 100 g dry yeast).
RESULTS
Human bronchial epithelial cell eotaxin-1 and mucin 5AC (MUC5AC) were markedly induced by the endotoxin LPS, which was dose-dependently attenuated by nontoxic dry-YE at 10-50 µg/mL. Moreover, dry-YE inhibited the MUC5AC induction enhanced by eotaxin-1, indicating that eotaxin-1-mediated eosinophilia may prompt the MUC5AC induction. Oral supplementation with 10-100 mg/kg dry-YE inhibited inflammatory cell accumulation in airway subepithelial regions with a reduction of lung tissue level of intracellular adhesion molecule-1. In addition, ≥ 50 mg/kg dry-YE diminished the lung tissue levels of eotaxin-1, eosinophil major basic protein and MUC5AC in OVA-exposed mice. Alcian blue/periodic acid schiff staining revealed that the dry-YE supplementation inhibited goblet cell hyperplasia and mucus overproduction in the trachea and bronchiolar airways of OVA-challenged mice.
The airway epithelium is a target of inflammatory and physical insults as well as an effecter of ongoing allergic inflammation [1]. Infiltration of inflammatory cells into the airway epithelium has been implicated in the pathophysiology of asthma, which is phenotypically characterized by eosinophilic and neutrophilic inflammatory patterns [12]. In general, eosinophilic inflammation is associated with the entire spectrum of severity of asthma, ranging from mild-to-moderate to severe pulmonary diseases [23]. Eosinophil infiltration into the bronchial mucosa results in allergic inflammation, epithelial remodeling and hyperresponsiveness in airways [45]. The recruitment of eosinophils into the lungs entails a cascade of processes directed by Th2 cytokines and multiple elements during allergic inflammation [567]. Defining the cellular mechanisms underlying the recruitment of eosinophils can propose potential targets for novel therapy [89]. Biological agents blocking Th2 inflammatory cascades have been developed for the management of severe asthma [1011]. In addition, novel small molecules such as chemokine receptor antagonists targeting neutrophilic inflammation reduce neutrophil infiltration [1213].
Mucus is overproduced in the respiratory tracts during acute challenges, as well as under chronic conditions such as asthma, chronic bronchitis and cystic fibrosis [1415]. Mucus obstruction is the result of several pathological processes by mucin gene regulation and secretion, as well as goblet cell hyperplasia and inflammatory mediators [14]. Goblet cells produce a constant overturning layer of protective mucus lining the airway epithelium [1617]. However, little is known about the pathophysiology of goblet cell hyperplasia in COPD, the treatment options are still limited, and there is currently no effective treatment [16]. Signaling pathways underlying upregulation of mucin synthesis and development of goblet cell hyperplasia are gradually being elucidated with various inflammatory mediators [18]. To date, Th2 cytokine pathways have been shown to have considerable potential for inhibition of excessive mucus production [1920]. Moreover, preclinical investigations have revealed clear potential targets for pharmacotherapy of airway mucus hypersecretion in asthma and COPD [2021].
Chronic mucus overproduction is associated with COPD exacerbations and increased risk of lung cancers [141522]. Accordingly, inhibition of mucus overproduction and obstruction by natural products is an imperative therapeutic target reducing critical airway complications. One investigation reported that the antioxidant sesamin attenuated eosinophil infiltration, airway goblet cell hyperplasia, mucus occlusion, and mucin 5AC (MUC5AC) expression in ovalbumin (OVA)-induced lung tissues [23]. However, little is known about beneficial effects of dietary yeast extract in goblet cell hyperplasia and mucus overproduction, and there is currently no effective yeast extract treatment. Therefore, the current study investigated whether glutathione-containing extracts from dry yeast (dry-YE) inhibited eosinophilia by reducing the eotaxin-1 induction in human bronchial cells and OVA-challenged mice. Moreover, this study evaluated whether dry-YE diminished eotaxin-1-mediated goblet cell hyperplasia and mucus overproduction through encumbering the increased MUC5AC induction.
M199, human epidermal growth factor (EGF), hydrocortisone, gelatin, human insulin, apotransferrin, chicken egg white albumin and LPS were obtained from Sigma-Aldrich Chemical (St. Louis, MO, USA), as were all other reagents, unless specifically stated otherwise. Fetal bovine serum (FBS), penicillin-streptomycin and trypsin-EDTA were purchased from Lonza (Walkersville, MD, USA). Eotaxin-1 (CCL11) antibody was purchased from R&D systems (Minneapolis, MN, USA) and β-actin antibody was obtained from Sigma-Aldrich Chemicals. Additionally, MUC5AC antibody was provided by Abcam (Cambridge, UK). Antibodies of toll-like receptor 4 (TLR4), intracellular adhesion molecule (ICAM)-1 and eosinophil major basic protein (EMBP) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG, donkey anti-goat IgG and goat anti-mouse IgG were purchased from Jackson Immuno-Research Laboratories (West Grove, PA, USA). Essential fatty acid free bovine serum albumin (BSA) and skim milk were supplied by Becton Dickinson Company (Sparks, MD, USA).
Dry-YE employed in the current study was provided by Mediense Co. Ltd (Chuncheon, Korea). Dry yeast was extracted by boiling in distilled water (10% w/v) at 40-60℃ for 24 h, followed by centrifugation at 3,000 rpm for 10 min. The generated supernatants were then harvested and passed through a 0.45 µm filter.
The dry-YE and L-glutathione (100 µL, 0.1 g in water) was incubated in 100 µL sodium carbonate (1 M, pH 9.8) and 100 µL of dansyl chloride solution (8 g/100 mL acetonitrile) in 500 µL distilled water at 80℃ for 40 min. After reaction, 100 µL acetic acid (50x dilution) was added and filtered with 0.45 µm for the HPLC analysis. Separation of L-glutathione was conducted on a reverse-phase Capcell Pak C18 UG 120 S5 column (Shiseido Co., Ltd, Tokyo, Japan) using an HPLC system (Shimadzu Co., Kyoto, Japan) at 40℃. The mobile phase was a binary elution of THF/methanol/50 mM sodium acetate (pH 6.2, 5 : 75: 420, A) and 100% methanol (B) under the following gradient conditions: initial, 20% B; 6 min, 20% B; 23 min, 100% B; 24 min, 20% B; 30 min, 20% B. The flow rate was 1.0 mL/min, and the injection volume was 10 µL. Eluted substances were detected at 210 nm with a photodiode-array detector.
Dry-YE was dissolved in dimethyl sulfoxide (DMSO) for live culture with cells. The final culture concentration of DMSO was < 0.5%.
The ROS production of was measured in 20 µM H2O2-exposed human alveolar basal epithelial A549 cells and human bronchial epithelial BEAS-2B cells using 2′-7′-dichlorofluorescein diacetate (DCF-DA), which is hydrolyzed and oxidized by ROS to a fluorescent compound 2′-7′-DCF. After lysation and incubation of lung tissue extracts with DCF-DA for 30 min, the fluorescence intensity was read in a Fluoroskan reader (Thermo Fisher Scientific, Waltham, MA, USA) with an appropriate filter.
The bronchial epithelial cell line BEAS-2B cells were provided by the American Type Culture Collection (Manassas, VA, USA). BEAS-2B cells were cultured in 25 mM HEPES-buffered M199 containing 10% FBS, 2 mM L-glutamine, 100,000 U/L penicillin, 100 µg/mL streptomycin supplemented with 2.5 µg/mL insulin, 361 ng/mL hydrocortisone, 2.5 µg/mL apotransferrin and 20 ng/mL EGF. BEAS-2B cells were sustained in 90-95% confluence at 37℃ in an atmosphere of 5% CO2. BEAS-2B cells were treated with 10-50 µg/mL dry-YE, and then stimulated with 2 µg/mL LPS or 20 ng/mL eotaxin-1 for up to 8 h in order to induce the expression of target gene proteins.
The toxicity of dry-YE was determined using 3-(4, 5-dimetylthiazol-yl)-diphenyl tetrazolium bromide (MTT, Duchefa Biochemie, Haarlem, Netherlands) after culture of BEAS-2B cells. These cells were incubated in fresh medium containing 1 mg/mL MTT for 3 h at 37℃. The purple formazan product was then dissolved in 0.5 mL isopropanol with gentle shaking, after which the absorbance of formazan was measured at λ = 570 nm using a microplate reader (BioRad Model 550, Hercules, CA, USA).
BEAS-2B cell lysates were prepared in 1 mM Tris-HCl (pH 6.8) lysis buffer containing 10% sodium dodecyl sulfate (SDS), 1% glycerophosphate, 0.1 mM Na3VO4, 0.5 mM NaF and protease inhibitor cocktail. Cell lysates containing equal amounts of proteins were electrophoresed on 8-15% SDS-PAGE, and then transferred onto a nitrocellulose membrane. Blocking of nonspecific binding was accomplished using either 3% fatty acid-free BSA or 5% non-fat dry skim milk for 3 h. The membrane was then incubated overnight at 4℃ with a specific primary antibody of TRL4, eotaxin-1 or MUC5AC, after which it was applied to a secondary antibody conjugated to HRP for 1 h. Following triple washing, the target proteins were determined using Immobilon Western Chemiluminescent HRP substrate (Millipore Corp., Billerica, MA, USA) and Agfa medical X-ray film (Agfa HealthCare NV, Mortsel, Belgium). Incubation with β-actin antibody was conducted as a comparative control.
Six week-old male BALB/c mice (Hallym University Breeding Center for Laboratory Animals) were used in the current study. Mice were kept on a 12 h light/12 h dark cycle at 23 ± 1℃ with 50 ± 10% relative humidity under specific pathogen-free circumstance, fed a nonpurified diet, and supplied with water ad libitum at the animal facility of Hallym University. Mice were acclimatized for 1 week before starting the experiments, at which time they were divided into five subgroups (n = 9 for each subgroup). Chronic allergic asthma was induced in murine models as described for previous studies [24]. Mice were then sensitized with 20 µg of ovalbumin (OVA) dissolved in 30 µL of phosphate buffered saline (PBS) with 50 µL of Imject Alum (Thermo Fisher Scientific, Rockford, IL, USA) through subcutaneous injections on days 0 and 14. Subsequently, 0.1 mL of dry-YE solution (containing 10-100 mg/kg BW) was administered via oral gavage to OVA-sensitized mice 1 h before challenge. On days 28-30, mice were subjected to 5% OVA inhalation for 20 min in a plastic chamber linked to an ultrasonic nebulizer (Clenny2 Aerosol, Medel, Italy). Control mice were then sensitized and challenged with PBS as the OVA vehicle. Twenty-four hours after the latest provocation (day 30), all mice were killed with an anesthetic (0.3 g/kg avertin and 8 µL/kg tert-amyl alcohol). The right lungs were kept at -80℃ for future Western blotting, while the left lungs were preserved and fixed in 4% paraformaldehyde and employed for immunohistochemical analyses. The current study was approved by the Hallym University Institutional Review Board and Committee on Animal Experimentation (Hallym 2016-11). This study was performed in compliance with the University's Guidelines for the Care and Use of Laboratory Animals. No dead mice were observed, and no evident signs of lethargy were detected during the experimental period.
To measure lung tissue levels of target proteins of eotaxin-1, MUC5AC, ICAM and EMBP, whole BALB/c lung tissue extracts were prepared in 1 mM Tris-HCl (pH 6.8) lysis buffer. Tissue extract containing an equal amount of proteins was electrophoresed for the Western blot analysis.
Paraffin-embedded tissue sections (5 µm thick) of airways and lungs were deparaffinized and hydrated to conduct immunohistochemical analyses. The sections were then pre-incubated in a boiling sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) for the antigen retrieval. Next, tissues were blocked with 5% BSA in PBS for 1 h. A specific primary antibody against eotaxin-1 or MUC5AC was incubated overnight with the sectioned tissues. Subsequently, the tissue sections were incubated for 1 h with FITC-conjugated anti-goat IgG or Cy3-conjugated anti-goat IgG. For identification of nuclei, the fluorescent nucleic acid dye DAPI was applied for 10 min. Stained tissues were then mounted on slides using mounting medium (Vector Laboratories, Burlingame, CA, USA). Images of each slide were obtained with an optical microscope Axioimager system (Zeiss, Gottingen, Germany).
To measure the acid and neutral mucins secreted from goblet cells, the paraffin-embedded lung specimens were sectioned to a thickness of 5 µm, deparaffinized, stained with alcian blue (Sigma-Aldrich Chemical) for 15 min, and then washed well in running tap water for 2 min. Subsequently, the tissue sections were PAS-stained and dehydrated with absolute alcohol. The stained tissue sections were then observed using an optical microscope Axioimager system and images were obtained for each section.
For the histological analyses, small airway specimens collected at the end of the experiments were fixed in 4% paraformaldehyde. The paraffin-embedded lung specimens were then sectioned at 5 µm, deparaffinized, stained using a modified Harris hematoxylin and eosin Y (H&E) for 2 min, and dehydrated through 50-100% absolute alcohol. The stained tissue sections were then observed using an optical microscope Axioimager system. Images were obtained for each section.
The results were expressed as the mean ± SE for each treatment group in each experiment. Statistical analyses were performed using the Statistical Analysis Systems statistical software package (SAS Institute, Cary, NC, USA). Significance was determined by one-way analysis of variance, followed by Duncan range test for multiple comparisons. Differences were considered significant at P < 0.05.
The dry-YE was evaluated for the quantitative and qualitative presence of glutathione. The HPLC spectra at λ = 210 nm showed that one peak was distinctly detected at the retention time of ≈13.0 min, and identified as glutathione with a yield of 140 mg/100 g dry weight (Fig. 1).
Cellular ROS production was significantly inhibited by treating H2O2-exposed A549 cells or BEAS-2B cells with 50 µg/mL dry-YE (Fig. 2A). The results indicated that dry-YE containing component glutathione (GSH) may act as a potent antioxidant antagonizing ROS production. In addition, there was no cytotoxicity observed in ≤ 50 µg/mL dry-YE-treated bronchial epithelial cells (Fig. 2B).
Western blot analysis revealed that TLR4 served as an epithelial receptor in response to LPS in the airway inflammatory process. The TLR4 expression was very weak in LPS-untreated quiescent cells, whereas it was induced in 2 µg/mL LPS-exposed bronchial epithelial cells (Fig. 2C). When epithelial cells were supplemented with ≥ 25 µg/mL dry-YE for 8 h, the TLR4 induction was significantly attenuated.
This study investigated whether treatment with dry-YE inhibited the induction of eotaxin-1 and MUC5AC in LPS-experienced bronchial epithelial cells. Eotaxin-1 expression was markedly elevated in LPS-elicited BEAS-2B cells, but was dose-dependently diminished by 10-50 µg/mL dry-YE (Fig. 2C). In addition, dry-YE nontoxic at ≤ 50 µg/mL suppressed the induction of the mucin protein MUC5AC by LPS in a dose-dependent manner (Fig. 2C). Furthermore, this study investigated whether airway eosinophilia was associated with airway mucus overproduction. As expected, 20 ng/mL eotaxin-1 greatly upregulated the airway epithelial MUC5AC expression (Fig. 2D). However, this induction was dampened by treating airway bronchial epithelial cells with 10-50 µg/mL dry-YE. Accordingly, eotaxin-1-mediated eosinophilic infiltration may be involved in LPS-induced mucin expression. The blockade of eotaxin-1 induction by dry-YE may entail the suppression of mucus overproduction in the airway epithelium.
Studies with cellular and animal models have demonstrated the roles of specific inflammatory cells of neutrophils, macrophages, and CD8+ T lymphocytes in COPD [25]. It can be assumed that eosinophil infiltration can activate neutrophils and alveolar macrophages [26]. Therefore, H&E staining was employed to reveal different cell infiltration in lung tissues between control mice and OVA-exposed mice. There were cardinal pathological features of asthmatic cell infiltration observed in the OVA-exposed mice. Specifically, OVA-sensitized and challenged mice displayed numerous inflammatory cells in the peribronchiolar and perivascular zones (Fig. 3A). In contrast, treatment with ≥ 50 mg/kg dry-YE markedly reduced the number of inflammatory cells infiltrating the peribronchiolar and perivascular regions. In addition, the OVA challenge elevated the lung tissue level of inflammatory ICAM-1 in mice, and oral administration of ≥ 50 mg/kg dry-YE reduced its expression in sensitized animals (Fig. 3B). These results are consistent in indicating persistent asthmatic inflammation induced by OVA exposure.
The current study attempted to determine whether dry-YE was effective at reducing the eotaxin-1 induction in OVA-challenged mouse airways. The lung tissue level of eotaxin-1 increased in mice sensitized and stimulated with 5% OVA (Fig. 4A). When 10-100 mg/kg dry-YE was orally administrated to OVA-challenged mice, the enhanced eotaxin-1 induction was significantly reduced. The immunohistochemical staining confirmed the induction of eotaxin-1 by the OVA challenge, which was evaluated by fluorescent microscopic observation using a specific eotaxin-1 antibody. Consistently, the eotaxin-1 expression (FITC-green staining) increased in bronchiolar epithelium and subepithelium of mice sensitized and stimulated with 5% OVA (Fig. 4B). When dry-YE was administrated to OVA-challenged mice, the induction of eotaxin-1 was markedly inhibited (Fig. 4C). Moreover, Dry-YE may encumber eosinophil infiltration in asthmatic airways, leading to structural changes in the bronchial airways. EMBP is implicated in epithelial cell damage and bronchospasm in allergic diseases and activates neutrophils and alveolar macrophages [26]. Western blot analysis showed that OVA exposure greatly increased the lung tissue level of EMBP, the predominant constituent in eosinophil granules (Fig. 4D). The oral administration of dry-YE diminished the EMBP level, indicating that dry-YE may alleviate eosinophilia in allergic asthma, leading to inhibition of the activation of neutrophils and alveolar macrophages.
Immunohistochemical staining was conducted to examine whether oral administration of 10-100 mg/kg dry-YE diminished the expression of MUC5AC in airways of OVA-challenged mice. There was heavy Cy3-red staining of MUC5AC in the epithelium and subepithelium of small bronchiolar airways (Fig. 5A). In contrast, oral supplementation of dry-YE markedly encumbered epithelial MUC5AC induction in OVA-challenged mice (Fig. 5B). Similarly, Western blot data revealed that 10-100 mg/kg dry-YE reduced the lung tissue level of MUC5AC elevated by OVA challenge (Fig. 5C). Accordingly, dry-YE may inhibit mucus overproduction in asthmatic airways.
Alcian blue-PAS staining is mainly used for staining mucus secreted from epithelial goblet cells. Alcian blue-PAS staining of small bronchiolar airways of OVA-exposed mice led to marked elevation in mucus staining, which was reversed by dry-YE treatment (Fig. 6A). As expected, there was a marked reduction of mucus-producing goblet cell staining observed in the trachea epithelium of dry-YE-treated OVA mice (Fig. 6B). Thus, dry-YE may reduce goblet cell hyperplasia and mucus production.
Six major findings were extracted from this study. 1) Dry-YE contained the antioxidant glutathione (GSH) at approximately 140 mg per 100 g dry yeast. 2) The endotoxin LPS markedly induced human bronchial epithelial cell eotaxin-1 and MUC5AC, which was dose-dependently attenuated by dry-YE nontoxic at 10-50 µg/mL. 3) The eotaxin-1-enhanced MUC5AC induction was inhibited in a dose-dependent manner by dry-YE. 4) Oral administration of 10-100 mg/kg dry-YE blocked inflammatory cell infiltration and reduced lung tissue levels of ICAM-1 in OVA-exposed mice. 5) Supplementation of dry-YE dampened the induction of eotaxin-1, EMBP and MUC5AC in OVA-exposed mouse airways. 6) Dry-YE inhibited goblet cell hyperplasia and mucus overproduction enhanced by OVA challenge in mouse trachea and bronchiolar airways. Therefore, glutathione-containing dry-YE can effectively ameliorate eosinophilic inflammation and allergic asthma by blocking oxidative stress-responsive induction of eotaxin-1 and MUC5AC.
The inflammatory infiltrates into airway regions implicated in the pathophysiology of asthma result in phenotypically disparate eosinophilic and neutrophilic inflammation [12]. Eosinophil infiltration into airways and inflammation is involved in epithelial remodeling and hypersensitivity typical of asthma [45]. The recruitment of eosinophils into the lungs results from a cascade of processes requiring Th2 cytokines and multiple elements including IL-5 and chemoattractants such as eotaxins and CCR3 during allergic inflammation [67]. This study showed that the endotoxin LPS highly upregulated eotaxin-1 in bronchial cells via prompting of TLR4. The binding of eotaxin to CCR3 expressed on eosinophils is essential to recruiting eosinophils [7]. Moreover, the induction of eotaxin-1 and EMBP was upregulated in OVA-exposed small airways. Thus, one can assume that the LPS episode and the OVA challenge resulted in the recruitment of eosinophils into the airways. The cellular events associated with eosinophil recruitment and inflammation will be the cornerstone of the development of novel therapies targeting pulmonary diseases such as asthma. In the current study, dry-YE blocked airway eosinophilia accompanying increased EMBP by inhibiting the eotaxin-1 induction by OVA challenge. On the other hand, the OVA challenge enhanced the inflammatory cell infiltration and ICAM-1 induction, thereby instigating asthmatic inflammation. In general, allergic sensitization occurs in more severe asthma via neutrophil recruitment [212]. Eosinophil infiltration can also activate neutrophils and alveolar macrophages [26]. This study revealed that inflammatory cells were infiltrated in airway subepithelial regions of OVA-challenged mice, but that this was inhibited by orally administrating dry-YE. A previous study showed that chemokine receptor antagonists targeting neutrophil infiltration reduce neutrophilic inflammation [13]. The inhibition of airway neutrophil recruitment by natural products such as dry-YE could be a strategy to combat allergic inflammation of asthma.
Therapies for asthma are based on the current understanding of the cellular mechanisms underlying eosinophilia and allergic inflammation. Endotoxins induce oxidative stress, resulting in eosinophilia and epithelial apoptosis in airways via activation of TLR4-PKCβ2-NADPH oxidase-responsive signaling [27]. In addition, oxidative stress controls key inflammatory signaling pathways and target proteins involved in airway and lung inflammation [28]. Accordingly, oxidative stress due to increased generation of reactive oxygen species (ROS) is a mechanistically imperative factor in eosinophilic inflammation in asthma. Knowledge of the mechanisms regulating ROS production could lead to the pharmacological manipulation of antioxidants in the inflammation and injury of airways and lungs [2930]. The dry-YE employed in this study contained a considerable amount of glutathione, which is a cellular antioxidant capable of preventing damage to important cellular components caused by ROS such as free radicals, peroxides, lipid peroxides, and heavy metals [31]. Thus, the inhibition of allergic inflammation and eosinophilia by dry-YE may be ascribed to its component glutathione. In fact, antioxidant polyphenolic compounds such as resveratrol, astragalin, kaempferol and sesamin provide protection against the damaging effects of oxidative stress, and thus may be useful in the management of inflammatory airways disease [23273233].
Mucus overproduction occurs in the respiratory tracts during acute episodes and in chronic conditions such as asthma, bronchitis and cystic fibrosis [1415]. The LPS exposure and OVA challenge highly induced bronchial and small airway MUC5AC protein, which was dampened by treatment of LPS-experienced bronchial epithelial cells and OVA-sensitized mice with dry-YE. Additionally, the MUC5AC induction in bronchial epithelial cells by LPS was directed at least by eotaxin-1, indicating that eosinophilia may be associated with mucus overproduction during allergic asthma. Inhibition of eotaxin-1-mediated eosinophilia by natural products would be a valuable therapeutic target for reducing mucus overproduction-linked airway complications. A previous study showed that the antioxidant sesamin inhibits eosinophil infiltration, MUC5AC expression, and mucus occlusion in OVA-induced exposed lung tissues [23]. Supplementation with lycopene, a potent dietary antioxidant, reduces eosinophilic infiltration and mucus-secreting cell numbers in the airways [34]. Similarly, the results of the present study indicate that dry-YE with glutathione as a potent antioxidant might attenuate eosinophil infiltration and mucus overproduction in OVA-exposed lung tissues.
Mucus hypersecretion is accompanied by goblet cell hyperplasia governed by inflammatory mediators such as IL-9 and IL-3 [1418]. Specific molecular pathways including Th2 cytokine pathways manipulating goblet cell differentiation and hyperplasia could be a prerequisite for the development of new therapeutic agents and a novel therapeutic approach for the treatment of asthma [1621]. OVA-induced airway hyperresponsiveness, airway eosinophilia and mucus hypersecretion are developed via the Th2-dependent JAK/STAT activation, downstream of the Th2 cytokine receptor signaling pathways [35]. However, there is the potential for development of undesirable side effects with pharmacotherapy, which would impede airway homeostasis. Isoimperatorin, an active natural furocoumarin, effectively suppresses airway inflammation and mucus hypersecretion by reducing the levels of Th2 cytokines and blocking NF-κB and MAPK pathways [36]. The current study showed that dry-YE blocked goblet cell hyperplasia and mucus overproduction in the trachea and small airways of OVA-challenged mice. Unfortunately, this study did not challenge specific mechanism(s) that may be involved in the inhibition of goblet cell hyperplasia by dry-YE. We also did not examine the effects of glutathione-alone on mucus overproduction. A previous study reported that glutathione alleviated pyocyanin-mediated modification of FOXA2, which is positively correlated with goblet cell hyperplasia and metaplasia, as well as with overexpression of airway mucin of MUC5AC and MUC5B [37]. Saucerneol D inducing heme oxygenase-1 inhibited eosinophilia and mucus hypersecretion through a marked decrease in ROS and malondialdehyde and an increase in superoxide dismutase and glutathione in lung tissues of OVA-sensitized mice [38]. Thus, glutathione present in dry-YE appeared to inhibit eosinophilia and mucus obstruction by suppressing oxidative stress in an OVA-induced asthma model.
In summary, this study investigated the potential for dry-YE to alleviate airway eosinophilia, goblet cell hyperplasia and mucus overproduction. Nontoxic dry-YE containing a significant amount of glutathione suppressed the endotoxin-induced expression of eotaxin-1 and MUC5AC in bronchial epithelial cells. The induction of MUC5AC by LPS could be caused by eotaxin-1-triggered eosinophilia, which was antagonized by treating epithelial cells with dry-YE. In addition, oral administration of glutathione-containing dry-YE dampened the eosinophilia, goblet cell hyperplasia and mucus overproduction observed in OVA-challenged airways. Therefore, oxidative stress may be involved in the induction of eotaxin-1 and MUC5AC by endotoxin episode and OVA challenge. Dry-YE effectively ameliorated oxidative stress-responsive epithelial eosinophilia and mucus-secreting goblet cell hyperplasia in cellular and murine models of asthma.
Figures and Tables
 | Fig. 1HPLC spectra at λ = 210 nm showing glutathione (GSH) detected in dry-yeast extracts (dry-YE).Glutathione peak UV spectrum at wavelengths of 200 to 800 nm (A), and chromatograms of glutathione (B) and dry-YE (C) with retention times, based on photodiode-array absorbance.
|
 | Fig. 2Inhibition of ROS production (A) and cytotoxicity of BEAS-2B cells by dry-yeast extracts (dry-YE) for 24 h (B), and blockade of induction of TLR4, eotaxin-1 and MUC5AC by dry-YE (C and D).BEAS-2B cells were cultured with 10-50 µg/mL dry-YE in the absence and presence of 2 µg/mL LPS or 20 ng/mL eotaxin-1. Cell viability was measured by MTT assay, and viability data are the mean ± SE (n = 4, cell viability of untreated controls = 100%). Cell lysates were prepared for Western blotting with a primary antibody against TLR4, eotaxin-1 and MUC5AC (C and D). β-Actin protein was used as an internal control. The bar graphs (mean ± SE, n = 3) represent quantitative results of the upper bands obtained from a densitometer. Means in bar graphs without a common letter differ, P < 0.05.
|
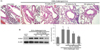 | Fig. 3H&E staining in small airways (A) and inhibition of ICAM-1 induction by dry-yeast extracts (dry-YE) in lung tissues of ovalbumin (OVA)-challenged mice (B).OVA-sensitized mice were orally administrated 10-100 mg/kg dry-YE. H&E staining was done in small airways of OVA-sensitized and dry-YE-treated mice (A). Each photograph is representative of four mice. Magnification: 400-fold. Tissue extracts were subject to Western blot analysis with an ICAM-1 antibody (B). β-Actin protein was used as an internal control. The bar graphs (mean ± SE, n = 3) represent quantitative results of the left bands obtained from a densitometer. Values in bar graphs not sharing a letter indicate a significant difference at P < 0.05.
|
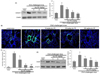 | Fig. 4Inhibitory effects of dry-yeast extracts (dry-YE) on eotaxin-1 induction in lung tissues of ovalbumin (OVA)-exposed mice.OVA-sensitized mice were orally administrated 10-100 mg/kg dry-YE. Tissue extracts were subject to Western blot analysis with a primary antibody of eotaxin-1 or EMBP (A and D). β-Actin protein was used as an internal control. The bar graphs (mean ± SE, n = 3) represent quantitative results of the left bands obtained from a densitometer. Immunohistofluorescence analysis showing inhibition of eotaxin-1 expression by dry-YE in OVA-challenged mouse small airways (B). The eotaxin-1 localization was visualized as FITC-green staining (B). Nuclear counter-staining was conducted with 4',6-diamidino-2-phenylindole (blue). Each photograph is representative of four mice. Magnification: 400-fold. The bar graphs (mean ± SE) represent quantitative results of FITC staining (C). Values in bar graphs not sharing a letter indicate a significant difference at P < 0.05.
|
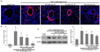 | Fig. 5Inhibition of MUC5AC induction by dry-yeast extracts (dry-YE) in lung tissues of ovalbumin (OVA)-exposed mice. OVA-sensitized mice were orally administered 10-100 mg/kg dry-YE.The MUC5AC localization was visualized as Cy3-red staining (A). Nuclear counter-staining was conducted with 4',6-diamidino-2-phenylindole (blue). Each photograph is representative of four mice. Magnification: 400-fold. The bar graphs (mean ± SE) represent quantitative results of FITC staining (B). Tissue extracts were subject to Western blot analysis with a MUC5AC antibody (C). β-Actin protein was used as an internal control. The bar graphs (mean ± SE, n = 3) represent quantitative results of the left bands obtained from a densitometer. Values in bar graphs not sharing a letter indicate a significant difference at P < 0.05.
|
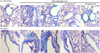 | Fig. 6Double staining of alcian blue and periodic acid schiff in small airways (A) and trachea (B) of ovalbumin (OVA)-exposed and dry-yeast extracts (dry-YE)-treated mice.OVA-sensitized mice were orally administered 10-100 mg/kg dry-YE. PAS and alcian blue staining was performed in small airways and trachea of OVA-sensitized and dry-YE-treated mice (arrows). Nuclear counter-staining was conducted with hematoxylin. Each photograph is representative of four mice. Magnification: 400-fold.
|
Acknowledgments
We thank Ms. Kyung-Hee Kim from Mediense Co. for providing dry yeast extracts and analyzing HPLC data.
References
1. Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011; 242:205–219.

2. Pelaia G, Vatrella A, Busceti MT, Gallelli L, Calabrese C, Terracciano R, Maselli R. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediators Inflamm. 2015; 2015:879783.

3. Possa SS, Leick EA, Prado CM, Martins MA, Tibério IF. Eosinophilic inflammation in allergic asthma. Front Pharmacol. 2013; 4:46.

4. Ohashi Y, Motojima S, Fukuda T, Makino S. Airway hyperresponsiveness, increased intracellular spaces of bronchial epithelium, and increased infiltration of eosinophils and lymphocytes in bronchial mucosa in asthma. Am Rev Respir Dis. 1992; 145:1469–1476.

5. Saitoh T, Kusunoki T, Yao T, Kawano K, Kojima Y, Miyahara K, Onoda J, Yokoi H, Ikeda K. Relationship between epithelial damage or basement membrane thickness and eosinophilic infiltration in nasal polyps with chronic rhinosinusitis. Rhinology. 2009; 47:275–279.

7. Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol. 2005; 175:5341–5350.

8. Kew KM, Quinn M, Quon BS, Ducharme FM. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Database Syst Rev. 2016; CD007524.

9. Beeh KM. The Role of Bronchodilators in preventing exacerbations of chronic obstructive pulmonary disease. Tuberc Respir Dis (Seoul). 2016; 79:241–247.

10. Papathanassiou E, Loukides S, Bakakos P. Severe asthma: anti-IgE or anti-IL-5? Eur Clin Respir J. 2016; 3:31813.

11. Patel TR, Sur S. IgE and eosinophils as therapeutic targets in asthma. Curr Opin Allergy Clin Immunol. 2017; 17:42–49.

12. Hosoki K, Itazawa T, Boldogh I, Sur S. Neutrophil recruitment by allergens contributes to allergic sensitization and allergic inflammation. Curr Opin Allergy Clin Immunol. 2016; 16:45–50.

13. Thomson NC. Novel approaches to the management of noneosinophilic asthma. Ther Adv Respir Dis. 2016; 10:211–234.

14. Rose MC, Nickola TJ, Voynow JA. Airway mucus obstruction: Mucin glycoproteins, MUC gene regulation and goblet cell hyperplasia. Am J Respir Cell Mol Biol. 2001; 25:533–537.

15. Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013; 187:228–237.

16. Boucherat O, Boczkowski J, Jeannotte L, Delacourt C. Cellular and molecular mechanisms of goblet cell metaplasia in the respiratory airways. Exp Lung Res. 2013; 39:207–216.

18. Lai H, Rogers DF. New pharmacotherapy for airway mucus hypersecretion in asthma and COPD: targeting intracellular signaling pathways. J Aerosol Med Pulm Drug Deliv. 2010; 23:219–231.

19. Takeyama K, Tamaoki J, Kondo M, Isono K, Nagai A. Role of epidermal growth factor receptor in maintaining airway goblet cell hyperplasia in rats sensitized to allergen. Clin Exp Allergy. 2008; 38:857–865.

20. Tanabe T, Kanoh S, Tsushima K, Yamazaki Y, Kubo K, Rubin BK. Clarithromycin inhibits interleukin-13-induced goblet cell hyperplasia in human airway cells. Am J Respir Cell Mol Biol. 2011; 45:1075–1083.

21. Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008; 70:487–512.

22. Bruse S, Petersen H, Weissfeld J, Picchi M, Willink R, Do K, Siegfried J, Belinsky SA, Tesfaigzi Y. Increased methylation of lung cancer-associated genes in sputum DNA of former smokers with chronic mucous hypersecretion. Respir Res. 2014; 15:2.

23. Lin CH, Shen ML, Zhou N, Lee CC, Kao ST, Wu DC. Protective effects of the polyphenol sesamin on allergen-induced T(H)2 responses and airway inflammation in mice. PLoS One. 2014; 9:e96091.

24. Gong JH, Shin D, Han SY, Kim JL, Kang YH. Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J Nutr. 2012; 142:47–56.

25. O'Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax. 2006; 61:448–454.
26. Swaminathan GJ, Myszka DG, Katsamba PS, Ohnuki LE, Gleich GJ, Acharya KR. Eosinophil-granule major basic protein, a C-type lectin, binds heparin. Biochemistry. 2005; 44:14152–14158.

27. Cho IH, Gong JH, Kang MK, Lee EJ, Park JH, Park SJ, Kang YH. Astragalin inhibits airway eotaxin-1 induction and epithelial apoptosis through modulating oxidative stress-responsive MAPK signaling. BMC Pulm Med. 2014; 14:122.

28. Lee IT, Yang CM. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol. 2012; 84:581–590.

29. Qu J, Li Y, Zhong W, Gao P, Hu C. Recent developments in the role of reactive oxygen species in allergic asthma. J Thorac Dis. 2017; 9:E32–E43.

30. Zuo L, Otenbaker NP, Rose BA, Salisbury KS. Molecular mechanisms of reactive oxygen species-related pulmonary inflammation and asthma. Mol Immunol. 2013; 56:57–63.

31. Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003; 66:1499–1503.

32. Wood LG, Wark PA, Garg ML. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid Redox Signal. 2010; 13:1535–1548.

33. Shin D, Park SH, Choi YJ, Kim YH, Antika LD, Habibah NU, Kang MK, Kang YH. Dietary compound kaempferol inhibits airway thickening induced by allergic reaction in a bovine serum albumin-induced model of asthma. Int J Mol Sci. 2015; 16:29980–29995.

34. Hazlewood LC, Wood LG, Hansbro PM, Foster PS. Dietary lycopene supplementation suppresses Th2 responses and lung eosinophilia in a mouse model of allergic asthma. J Nutr Biochem. 2011; 22:95–100.

35. Ashino S, Takeda K, Li H, Taylor V, Joetham A, Pine PR, Gelfand EW. Janus kinase 1/3 signaling pathways are key initiators of TH2 differentiation and lung allergic responses. J Allergy Clin Immunol. 2014; 133:1162–1174.

36. Wijerathne CUB, Seo CS, Song JW, Park HS, Moon OS, Won YS, Kwon HJ, Son HY. Isoimperatorin attenuates airway inflammation and mucus hypersecretion in an ovalbumin-induced murine model of asthma. Int Immunopharmacol. 2017; 49:67–76.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download