INTRODUCTION
In recent years, numerous researches have reported that the excessive rise in blood glucose levels after a meal increases the risk of developing chronic and metabolic diseases, whereas low-glycemic diets and foods reduce the risk [
12]. Conventional carbohydrates utilized in the food industry such as processed starch and glucose syrups stimulate a high glycemic response [
3]. In particular, sucrose is a disaccharide that is composed of glucose and fructose and is used in a variety of applications as a sweetener. Sucrose is also used as a standard for sweetness in the food industry. Following the rapid hydrolysis of sucrose into glucose and fructose in human intestines, an increase in blood glucose level occurs. Thus, excessive intake of sucrose can increase an individual's susceptibility to diseases such as tooth decay, obesity, and type II diabetes [
4]. For individuals with diabetes mellitus, maintenance of moderate blood glucose levels is important. And the modification of the glycemic effect of food by substitution of these high glycemic carbohydrate with low glycemic sugar alternatives has been important issue in the food industry. Consequently, the development of low calorie and low glycemic sweeteners can potentially help to prevent or manage related metabolic diseases.
One of the sucrose isomers that naturally exist in honey is turanose, 3-O-α-D-glucosyl-D-fructose. The sweetness of turanose is half of sucrose [
56] and it is hydrolyzed more slowly than sucrose. Thus, turanose could potentially serve as a low glycemic sweetener similar to palatinose (isomaltulose) [
7]. Turanose is a byproduct of the synthesis of linear α-(1,4)-glucan from sucrose, a reaction that is catalyzed by amylosucrase. A recombinant form of this enzyme has been generated based on the amylosucrase enzyme in
Neisseria polysaccharea [
8]. When α-cyclomaltodextrin is combined with D-fructose from
Bacillus stearothermophilus and glucoamylase, the yield of turanose is approximately 45% [
6]. This production yield of turanose can be increased to 73.7% when extrinsic fructose and sucrose at a high concentration are provided as substrates in an amylosucrase reaction [
7]. The hydrolysis rate of turanose has been reported to be approximately 54% of sucrose and 6% of maltose using a 5 mM in a rat intestinal enzyme mixture [
910]. There is lack of evidence regarding the hydrolysis and absorption of turanose in human small intestine. However, turanose was hydrolyzed by 13% with
in vitro human intestinal biopsy sample [
11]. Thus, turanose has the potential to be low glycemic alternative sweetener similar to palatinose [
12].
Previously, the treatment of pre-adipocytes with turanose resulted in attenuation of glucose-induced lipid accumulation [
7]. Based on the role of turanose in controlling adipogenesis and in the enzymatic process of turanose production, turanose represents a potential sweetener that can replace sucrose. However, toxicological aspect of administrating turanose at high doses needs to be confirmed in animal models.
In the present study, acute and subchronic oral toxicity studies of turanose were conducted with ICR mice. These results provide an important reference for the use of turanose in biomedical applications or as a commercial substitute for sucrose.
MATERIALS AND METHODS
Materials
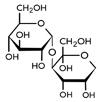
Turanose (3-O-α-D-glucosyl-fructose; CAS No. 547-25-1) was purchased from Carbosynth Limited (Berkshire, United Kingdom) and its structure was presented in
Fig. 1. Turanose was freshly prepared as a solution in distilled water and was orally administered via gavage in a volume of up to 1 mL/100 g body weight (b.w.).
Animals
ICR mice (7 weeks, weighing approximately 34 g for male and 29 g for female) were used for acute toxicity study and ICR mice (5 weeks, approximately 28 g for male and 27 g for female) were used for subchronic toxicity studies, respectively. All of the mice were purchased from Central Lab, Animal Inc. (Seoul, Korea) and were acclimated to laboratory conditions for 7 d. The mice were housed individually in suspended plastic cages to monitor individual food intake, water intake, and body weight changes for each mouse. Drinking water and food [American Institute of Nutrition (AIN)-93G diet (Unifaith Inc., Seoul, Korea)] were provided ad libitum. The mice were maintained in conditions of 22-25℃, 50-60% relative humidity, a 12 h light/dark cycle, and approximately ten air changes per hour. Both the acute and chronic oral animal toxicity study protocols were reviewed and approved by the Ewha Institutional Animal Care and Use Committee (IACUC) (No. 16-005 and No. 16-006).
Acute oral toxicity study
Following the administration of a single maximal feasible oral 10 g/kg b.w. of turanose on male and female ICR mice, mortality and adverse effects were evaluated. The test was performed according to the procedures outlined in the OECD guideline for testing chemicals (No. 420), with the exception that the dose was 10 g/kg b.w./day instead of the 5 g/kg b.w./day recommended in the guideline [
13]. After a 7-day period of acclimatization, 20 ICR mice were randomly assigned into four groups of 5 mice based on body weight.
Initially, a sighting study was conducted prior to the main study. One animal of each gender was fasted overnight while receiving drinking water ad libitum. Both mice were then administered 10 g/kg b.w. of turanose one day prior to the initiation of the main study. Clinical and behavior signs of the two animals were monitored for the first 1 h after the administration of turanose and every hour subsequently for at least 6-24 h. After confirming the survival of the two animals in the sighting study for 24 h, an additional 20 mice were administered turanose for the main study.
Briefly, 20 ICR mice were fasted overnight before being weighed. The test groups received a single oral administration of turanose (10 g/kg b.w.), while the same volume of water was orally administered to the control group of each sex. All of the animals were observed for 30 min after the administration of turanose or water, then every hour for the first 6 h, and then periodically for the remainder of the first 24 h. After 24 h, daily clinical signs of toxicity such as tremors, convulsions, salivation, diarrhea, lethargy, sleep, and coma were recorded. The body weight of each mouse was recorded on the first day of oral administered of turanose and twice a week for 14 days. Final measurements of body weight, food, and water consumption were made after the animals were fasted overnight prior to sacrifice.
On day 15, mice were anesthetized with isoflurane (Piramal Critical Care, Bethlehem, PA, USA) before various organs were resected and weighed, including brain, pituitary gland, heart, lung, liver, spleen, kidney, adrenal gland, testis, prostate gland, and ovary. The relative organ weights by b.w. were calculated and statistically analyzed. The median lethal oral dose (LD50) (mg/kg) was calculated based on the number of animals that died during the study.
Subchronic (13 weeks) toxicity study
A subchronic toxicity study was conducted for 13-week feeding study. Briefly, mice were acclimatized to laboratory condition for 1 week. Then, forty 5-week-old ICR mice (20 males and 20 females) were randomized into 4 male groups and 4 female groups to receive turanose at doses of 0, 1.75, 3.5, and 7 g/kg b.w. via gavage with 5 mice per group. The highest dose administered was selected based on the total amount of sugar consumed by mice in their diet (e.g. 10% of their diet).
Animals were monitored for signs of toxicity and mortality during a 13-week feeding period. The body weight of each animal was recorded on the first day of turanose administration and then twice a week throughout the experimental period. Food and water consumption were also measured twice a week for the duration of the study. After 13 weeks, the mice underwent fasting overnight before being anesthetized and sacrificed. Various organs (as listed for the acute study) and blood samples were collected from the abdominal aorta. The serum was separated by centrifugation at 3000 rpm for 15 min before being analyzed.
Clinical observations of signs of toxicity, changes in body weight, and food and water consumption in subchronic toxicity study
Clinical behaviors and abnormal signs of toxicity including tremors, convulsions, salivation, diarrhea, lethargy, sleep, and coma were observed daily during the test period. Individual body weights were recorded twice a week just prior to administration of turanose. The final body weights and amounts of food and water consumption were recorded before the mice were sacrificed. The no-observed-adverse-effect-level (NOAEL) of turanose in the ICR mice was calculated based on the survival rate, observations, and administered doses.
Relative organ weights and macroscopic necropsy in subchronic toxicity study
After 13 weeks of administrating turanose daily, a complete necropsy was conducted on all of the animals following euthanasia by isoflurane inhalation. The absolute weights and relative organ weights of the following organs in relation to the final body weights (100 g) were recorded for toxicity: brain, pituitary gland, heart, lung, liver, spleen, kidney, adrenal gland, testis, prostate gland, and ovary.
Histopathological analysis in subchronic toxicity study
At the time of necropsy, various organs were collected from both sexes of the groups and these were fixed in 10% neutral buffered formalin and processed into paraffin blocks. Sections (4-5 um) were sliced, and mounted on glass slide and stained with hematoxylin-eosin. Two independent pathologists examined the sections with light microscopy (Olympus, Co., Tokyo, Japan)
Hematology and serum biochemistry in subchronic toxicity study
Hematological parameters, including hematocrit, hemoglobin concentrations, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red blood cell (RBC) count, white blood cell (WBC) count, and platelet count were analyzed in whole blood samples by using an XN9000 system (SYSMEX, Kobe, Japan).
Biochemical parameters of the serum samples were measured using a Modular Analytics System (Hitachi Ltd., Tokyo, Japan) and commercial kits from Roche Deutschland Holding GmbH (Mannheim, Germany). Biochemical parameters, including levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total protein, albumin, total bilirubin (TB), blood urea nitrogen (BUN), creatinine, glucose, triglyceride, total cholesterol (TC), calcium, chloride, and potassium were analyzed.
Statistical analysis
All data are presented as the mean ± standard deviation (SD) using GraphPad PRISM software (GraphPad Software, San Diego, CA, USA). For the acute toxicological study, data from the test group were compared with data from the control group according to gender with an unpaired Student's t-test (two-tailed). The data from the subchronic toxicity test were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test. A P-value less than 0.05 was considered statistically significant.
DISCUSSION
Previously, the potential for turanose to serve as an alternative sweetener was proposed based on the anti-adipogenic effect and low-glycemic property [
7]. Moreover, both of these properties represent potential health benefits. In the present study, a comprehensive safety evaluation was performed for turanose which included both acute and 13-week subchronic oral toxicity studies in male and female ICR mice. No toxicity was observed in either study and the NOAEL was calculated to be 7 g/kg/day.
For the acute toxicological study, the administered dose (10 g/kg b.w.) was selected based on a previous toxicological study that was conducted for another analogue, palatinose (isomaltulose), which is an isomer that is similar isomer to sucrose [
1415]. In the study by Jonker et al. [
15], doses of palatinose up to 8.1 g/kg b.w./day did not result in adverse effects. Correspondingly, in the sighting experiment conducted in the present study, no adverse signs were observed following the oral administration of 10 g/kg b.w. turanose to both a male and a female ICR mouse (data not shown). In the main acute study, all of the mice survived without any abnormalities during the 14-day study and the LD
50 for oral intake of turanose was calculated to be greater than 10 g/kg b.w. Thus, the present results confirm that the administration of a single oral dose of turanose up to 10 g/kg b.w. for 14 days is safe.
In the subchronic toxicological study, turanose was administered to ICR mice via gavage at doses of 0, 1.75, 3.5, and 7 g/kg b.w. based on the results of the acute toxicity test and a previous subchronic toxicity study of palatinose [
15]. In the absence of any turanose-related deaths or clinically abnormal behavior among all the treatment groups throughout the subchronic toxicity study, the NOAEL was calculated to be greater than 7 g/kg b.w./day for both the male and female ICR mice. In addition, there were no significant changes in body weights for any of the treatment groups. A decrease in body weight was observed for the male mice in the 7 g/kg b.w. group during the study; however, the final mean body weight for the males in the 7 g/kg b.w. group did not differ from that of the control group. A similar result was observed for female groups. Since there have been no comparative studies of the effects of different doses of turanose on the body weight of mice to date, the changes in body weight observed in the present study should be confirmed in future studies.
During a few weeks of the current study, food consumption differed for the male mice. However, these results were not considered toxicologically relevant to turanose since the differences were minimal and these were not consecutively observed. Similarly, although the relative organ weights of some of the organs examined exhibited a consistent decrease or increase, particularly the spleen and brain in male mice and the liver and kidney in the female mice, respectively, no significant differences were observed in any of the organs. In addition, the observed trends were not consistent between the male and female mice. Therefore, it is proposed that no toxicologically significant changes were caused by the administration of turanose in the mouse model examined.
In the histopathological examinations performed, no differences between the control and 7 g/kg b.w. turanose groups were observed. For example, there were lesions present in liver and kidney tissues in both the control and treatment groups. In the hematological analysis, although the mean RBC value in the male mice of the 1.75 g/kg b.w. turanose group was significantly higher than that of the control group, this significance was considered to be a transient alteration and not a relevant toxic effect since the higher dose groups did not exhibit any significant differences in RBC values from the control mice. Based on these results, although it was not significant, most of the turanose-treated mice tended to have elevated glucose levels in both the male and female mice compared to the control groups in both males and females. This result should be noticed as the possibility that turanose elevates blood glucose levels. However, the levels of blood glucose and TG tended to be lower in male 7g/kg b.w. turanose treatment group compared to 1.75 g/kg b.w. turanose group. Turanose is hydrolyzed more slowly than sucrose [
7] and replacement of sucrose with turanose lowered blood glucose level in high fat-diet induced obese mice (unpublished data). Previously, turanose at cellular levels suppressed preadipocyte differentiation, and thus exerted a potential health benefit to prevent obesity and related metabolic diseases [
7]. Since the absorption rate of turanose remains to be determined, further studies related to the metabolism of turanose and the effect of turanose on regulating glucose homeostasis are needed and recommended.
The present results demonstrate that oral administration of turanose as a single dose or over an extended period of time did not cause any noticeable adverse effects in ICR mice. By applying a conversion rate for mouse to human (12.33) [
16], the human equivalent dose (HED) for the treatment concentration (7 g/kg b.w.) would be 568 mg/kg. Furthermore, the acceptable daily intake (ADI) for turanose for humans, based on application of a safe factor of 100 to the NOAEL, would be 70 mg/kg/day according the present study.
Despite the absence of any observed toxicities in the present model, it is possible that administration of turanose may be associated with side-effects that were not examined in this study. For example, side effects involving reproduction/developmental processes, gene mutagenesis, carcinogenic processes, and inhalation toxicity were not investigated. Therefore, further toxicological and functional studies are needed to confirm the safety and efficacy of establishing turanose as a sugar substitute.
In conclusion, to our knowledge, this is the first study to evaluate acute and subchronic toxicity profiles for turanose in ICR mice. The 14-day acute oral toxicological study showed no toxic effects, and an LD50 value was greater than 10 g/kg b.w. for a single dose of oral administration of turanose. Similarly, no noticeable toxicological effects were observed in any of the treated groups over the 13-week subchronic oral toxicological study. The NOAEL calculated to be greater than 7 g/kg/day for both male and female ICR mice. In addition, the ADI of turanose was calculated to be 70 mg/kg/day. Taken together, these results suggest that a high-dose of turanose is safe for both short-term and long-term administration.












 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download