Abstract
BACKGROUND/OBJECTIVES
MATERIALS AND METHODS
RESULTS
Figures and Tables
 | Fig. 1Effects of KME on antioxidative transcription and enzymes in the aorta of LDL receptor knockout mice fed a high cholesterol diet for 8 weeks.Data are the mean ± SD (n = 10 per group). See the legend in Table 1 for descriptions of the control and KME groups. *Significant differences between two experimental groups are expressed as P-values calculated by Student's t-test (P < 0.05). KME, kimchi methanol extracts; Nrf2, nuclear factor (erythroid-derived 2)-like 2; SOD, superoxide dismutase; CAT, catalase; GSHPx, glutathione peroxidase.
|
 | Fig. 2Effects of KME on endoplasmic reticulum stress in the aorta of LDL receptor knockout mice fed a high cholesterol diet for 8 weeks.Data are the mean ± SD (n = 10 per group). See the legend in Table 1 for descriptions of the control and KME groups. *Significant differences between two experimental groups are expressed as P-values calculated by Student's t-test (P < 0.05). KME, kimchi methanol extracts; GRP78, glucose regulated protein 78; p-PERK, phospho-protein kinase RNA-like ER kinase; p-eIF2α, phospho-eukaryotic initiation factor 2 subunit alpha; XBP1, X-box binding protein 1; CHOP, C/EBP homologous protein.
|
 | Fig. 3Effects of KME on apoptosis-related molecules in the aorta of LDL receptor knockout mice fed a high cholesterol diet for 8 weeks.Data are the mean ± SD (n = 10 per group). See the legend in Table 1 for descriptions of the control and KME groups. *Significant differences between two experimental groups are expressed as P-values calculated by Student's t-test (P < 0.05). NS, no significance; KME, kimchi methanol extracts; p-JNK, phospho-c-Jun N-terminal kinase; Bax, bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; cIAP, cellular inhibitor of apoptosis protein.
|
 | Fig. 4Oil red O and TUNEL staining of the aortic sinus of LDL receptor knockout mice fed a high cholesterol diet for 8 weeks.Data are the mean ± SD (n = 10 per group). Representative sections were stained with oil red O (magnification 40×) and TUNEL (magnification 400×). The apoptotic index was calculated by the following formula: 100 × [number of TUNEL-positive cell nuclei/total number of cell nuclei]. Arrows indicate TUNEL-positive cells. *Significant differences between two experimental groups are expressed as P-values calculated by Student's t-test (P < 0.05). KME, kimchi methanol extracts; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end labeling.
|
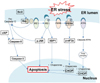 | Fig. 5Mechanism for ER stress and apoptosis elucidated in this study.ER, endoplasmic reticulum; Bcl-2, B-cell lymphoma 2; Bax, bcl-2-associated X protein; IRE1, inositol requiring kinase 1; PERK, protein kinase RNA-like ER kinase; ATF6, activating transcription factor 6; cIAP, cellular inhibitor of apoptosis protein; p-JNK, phospho-c-Jun N-terminal kinase; XBP1, X-box binding protein 1; p-eIF2α, phospho-eukaryotic initiation factor 2 subunit alpha; CHOP, C/EBP homologous protein.
|
Table 1
Body weight, food intake, and food efficacy of low-density lipoprotein receptor knockout mice fed a high cholesterol diet with distilled water or kimchi methanol extracts (KME) for 8 weeks

Data are the mean ± SD (n = 20 per group).
1)The control group was fed a high cholesterol diet (HCD) with oral administration of distilled water for 8 weeks. The KME group was fed a HCD with oral administration of kimchi methanol extracts (200 mg·kg-bw−1·day−1) for 8 weeks.
2)Total weight gains were divided by total food intake.
3)The food efficacy ratio is expressed as the total weight gain/total food intake.
NSdata in the column are not significantly different.
Table 2
Biochemical analysis data of plasma lipid levels in low-density lipoprotein receptor knockout mice fed a high cholesterol diet with distilled water or kimchi methanol extracts (KME) for 8 weeks

Data are the mean ± SD (n = 20 per group).
1)See the legend in Table 1 for descriptions of the control and KME groups.
2)LDL-C was calculated as TC-HDL-C-(TG/5).
3)The atherogenic index was calculated as (TC-HDL-C)/HDL-C.
4)Reactive oxygen species (ROS) and peroxynitrite ratios are expressed as the respective value relative to the control group.
*Data between the two groups are significantly different by Student's t-test (P < 0.05).
TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download