Abstract
BACKGROUND/OBJECTIVES
We investigated the anti-osteoarthritic effects of deer bone extract on the gene expressions of matrix metalloproteinases (MMPs) and collagen type II (COL2) in interleukin-1β-induced osteoarthritis (OA) chondrocytes.
MATERIALS/METHODS
Primary rabbit chondrocytes were treated as follows: CON (PBS treatment), NC (IL-1β treatment), PC (IL-1β + 100 µg/mL glucosamine sulphate/chondroitin sulphate mixture), and DB (IL-1β + 100 µg/mL deer bone extract).
RESULTS
The results of the cell viability assay indicated that deer bone extract at doses ranging from 100 to 500 µg/mL inhibits cell death in chondrocytes induced by IL-1β. Deer bone extract was able to significantly recover the mRNA expression of COL2 that was down-regulated by IL-1β (NC: 0.79 vs. DB: 0.87, P < 0.05) and significantly decrease the mRNA expression of MMP-3 (NC: 2.24 vs. DB: 1.75) and -13 (NC: 1.28 vs. DB: 0.89) in OA chondrocytes (P < 0.05).
CONCLUSIONS
We concluded that deer bone extract induces accumulation of COL2 through the down-regulation of MMPs in IL-1β-induced OA chondrocytes. Our results suggest that deer bone extract, which contains various components related to OA, including chondroitin sulphate, may possess anti-osteoarthritic properties and be of value in inhibiting the pathogenesis of OA.
Osteoarthritis (OA) is a complex degenerative disease of the joints characterised by the pathological features of trabecular bone, and accompanied with pain as the most prominent and debilitating symptom [12]. Although a number of risk factors have been identified for pain in OA, the research focus has primarily been on structural targets. Pharmacologic treatment options remain limited and non-pharmacologic options are underutilized [3]. An expansion of the research agenda to more fully explore pain mechanisms in OA is urgently needed to enable comprehensive mechanism-based pain management strategies in this common, disabling, and costly disease.
The administration of non-steroidal anti-inflammatory drugs (NSAIDs) is the conventional treatment for OA. These medications often relieves OA symptoms but possess serious side effects [4]. Many people with OA try complementary and alternative therapies, so efforts are being made to define the role of natural products for OA treatment [45]. One of the most common alternative medication for treatment of OA is chondroitin sulphate, a sulphated glycosaminoglycan composed of a chain of alternating sugars (N-acetylgalactosamine and glucuronic acid). Chondroitin sulphate is an important structural component of cartilage and provides much of its resistance to compression [6].
Deer bone is a possible new natural source of chondroitin sulphate, and it contains other components related to cartilage, such as glucosamine, calcium, ganglioside, and collagen, in addition to chondroitin sulphate [7]. Therefore, deer bone can be useful as a natural supplement in OA treatment without serious side effects. However, the pharmacological actions of deer bone on OA have not been well documented. We investigated the gene expressions related to cartilage degradation in OA chondrocytes treated with deer bone extract to clarify the anti-osteoarthritic effects of deer bone. In this study, chondrocytes were collected from slices of rabbit articular cartilage, then treated with interleukin (IL)-1β to induce OA in the chondrocytes.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), collagenase, and recombinant human IL-1β were purchased from Sigma Chemical Co. (MO, USA). Fetal bovine serum (FBS), antibiotic/antimycotic solution, penicillin streptomycin, and Dulbecco's modified Eagle's medium (DMEM) were purchased from Gibco BRL (NY, USA). TRIzol LS reagent, oligo-dT, and superscript II reverse transcriptase were purchased from Invitrogen Co. (CA, USA). All other reagents were of the highest commercial grade available.
Dried deer bone from adult male Russian elks was obtained from Nongshim Co. (Seoul, Korea). The upper part of the deer bone (100 g) was extracted three times with 1 L of distilled water, followed by reflux for 5 h, and cooling. The extract was centrifuged at 3,000 × g for 20 min. The supernatants were concentrated using a vacuum evaporator at 40℃ and lyophilised.
For amino acid analysis, samples were hydrolyzed with 6 N HC1 at 110℃ for 24 h. Hydroxyproline content in the hydrolysate was determined by the method of Stegemann and Stalder [8]. The collagen content was calculated by multiplying the content of hydroxyproline by 7 [9].
The total ganglioside content was measured as µg N-acetylneuraminic acid (Neu5Ac)/1 g [10]. Neu5Ac were determined by HPLC-fluorescence detection following derivatization with DMB according to the method described by Hara et al. and Salcedo et al. [1112]. The sulfated GAGs (Chondroitin sulfate) content was determined using the DMMB solution by the method of Barbosa et al. [13].
Chondrocytes were released by enzymatic digestion from slices of articular cartilage taken from 8-week-old male white New Zealand rabbits (Samtako Biokorea Co., Osan, Korea) [14]. The cartilage was collected in DMEM with high glucose and was sliced into pieces of 2 to 3 mm2 in 40 mL culture medium (DMEM/high glucose, 2% FBS, 1% antibiotic/antimycotic solution, and 1% penicillin/streptomycin) containing 0.2% collagenase. The cartilage was shaken on an orbital shaker at 50 rpm, and incubated at 37℃ for 5 h. The digestate was suspended and filtered through a 70 cm nylon cell strainer. Cells were centrifuged at 1,000 × g for 10 min. The supernatant was removed, and the pellet was gently resuspended in 40 mL of culture medium. The resuspended pellet was placed into cell culture flasks at a concentration of 1.5 × 104 cells/mL. The glucosamine sulphate/chondroitin sulphate mixture was used as a positive control to compare the effect of deer bone extract. Chondrocytes were treated with phosphate buffered saline (PBS) or 25 ng/mL IL-1β before receiving the following treatments: CON (PBS treatment); NC (IL-1β treatment); PC (IL-1β + 100 µg/mL glucosamine sulphate/chondroitin sulphate mixture); DB (IL-1β + 100 µg/mL deer bone extract).
Cell viability was measured by MTT assay based on the method of Alley et al. [15] with some modifications. Cells were treated with deer bone extract at a final concentration of 0, 50, 100, 250, or 500 µg/mL with or without 25 ng/mL IL-1β. They were then treated with 1 mg/mL MTT solution and incubated in a humidified, 95% air, 5% CO2 atmosphere at 37℃ for three hours. The formed formazan crystals were extracted with 10% SDS in 1 N HCl and measured at 540 nm on a microplate reader. Data were expressed as the mean percentage of viable cells compared to the control.
RNA isolation was determined by the method of Marlovits et al. [16] with some modifications. Complementary DNA (cDNA) was synthesised from the isolated RNA by oligo-dT and superscript II reverse transcriptase. Polymerase chain reaction (PCR) was performed according to the manufacturer's protocol (Koma Biotech Inc., Seoul, Korea). PCR products were separated by electrophoresis on a 1% agarose gel. The bands separated on the agarose gel were visualised using Fluor Chem FC2 (Alpha Innotech Co., CA, USA). The intensity of the bands was measured using Alpha view software (Alpha Innotech Co.). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an internal control to determine the relative values of RNA expression [17]. Values were expressed in arbitrary units with normalization with GAPDH expression. The sequences of the target gene primer pairs that were used for PCR are listed in Table 1.
The statistical analysis was performed with the Statistical Package for Social Sciences (SPSS, Inc., an IBM Company, Chicago, Illinois, USA). The results were expressed as the means ± SD of experiments performed in triplicate. The cell viability for chondrocytes treated with IL-1β was compared to that for cells without IL-1β treatment and the differences were analysed by t-test. For gene expression, the differences among treatments were analysed using one way analysis of variance (ANOVA) by Tukey's multiple range tests. Null hypotheses of no difference were rejected if P-values were less than 0.05.
The deer bone extract composed of crude protein (80.9%), crude lipid (12.5%), crude ash (3.5%) and moisture (3.1%) (data not shown). Table 2 shows the hydroxyprolin, collagen, gaglioside and chondroitin sulfate content of deer bone extract. Hydroxyproline and collagen contents are 11.36 mg/g and 79.73 mg/g. Hydroxyproline is one of three predominant amino acids constituting collagen, a major protein in deer bone extract. Ganglioside is an important component in velvet antler and serves as the quality index of velvet antler [18]. Therefore, we also determined the ganglioside (1.03 mg/g) and chondroitin sulfate (73.74 µg/g).
Fig. 1 shows the effect of deer bone extract on the cell viability of OA chondrocytes treated with IL-1β. These results show that IL-1β significantly decreases the cell viability of chondrocytes (P < 0.05). After exposure to IL-1β, the percentage of viable chondrocytes decreased significantly to 84.0% compared to the IL-1β-untreated chondrocytes (P < 0.05). Thus, a loss of cell viability (16%) occurred when chondrocytes were treated with IL-1β. When chondrocytes were treated with deer bone extract doses ranging from 100 to 500 µg/mL, there was a significant restoration of the decreased cell viability that was induced by IL-1β (P < 0.05).
Fig. 2 presents the effect of deer bone extract on the mRNA expression of COL2 in IL-1β-treated chondrocytes. IL-1β induced a significant decline in the mRNA expression of COL2 (CON: 1.00 vs. NC: 0.79, P < 0.05). A significant increase in the mRNA expression of COL2 was observed with deer bone extract treatment (DB: 0.87) compared to non-treatment (NC: 0.79) in IL-1β-treated chondrocytes (P < 0.05).
To evaluate the effects of deer bone extract on the mRNA expression of MMP-1, -3, and -13 up-regulated by IL-1β, chondrocytes were incubated with or without deer bone extract after IL-1β treatment. These results are summarised in Fig. 3, 4, and 5. The mRNA expression of MMPs in IL-1β-treated chondrocytes (NC) was significantly higher than that in IL-1β-untreated chondrocytes (CON) as shown in Fig. 3, 4, and 5 (P < 0.05). The mRNA expression of MMP-3 (NC: 2.24 vs. DB: 1.75) and -13 (NC: 1.28 vs. DB: 0.89) in OA chondrocytes declined especially significantly with deer bone extract treatment (P < 0.05). Deer bone extract also tended to restore the mRNA expression of MMP-1 up-regulated by IL-1β, but a significant difference between NC and DB was not observed.
An in vitro cytotoxicity test is important to provide the data for safety assessment of a specific sample. In vitro cytotoxicity and in vivo safety are highly associated [19]. In this study, deer bone extract was not toxic to chondrocytes in doses ranging from 0 to 500 µg/mL (Fig. 1). Almost no adverse effects on in vitro chondrocyte viability were observed from treatment with deer bone extract. IL-1β significantly decreased the cell viability of chondrocytes. There have been reports that IL-1β induces the death of chondrocytes. Yasuhara et al. [20] demonstrated progressive cell death as well as mitochondrial dysfunction following IL-1β treatment in chondrocytes. As shown in Fig. 1, deer bone extract in doses ranging from 100 to 500 µg/mL inhibited the cell death of chondrocytes induced by IL-1β.
Collagens provide tensile strength and shock absorption. COL2 is the most abundant domain among cartilage collagens. Degradation of these collagens contributes to the destruction and loss of function of cartilage in arthritic disease [21]. The mechanism of cartilage destruction is under investigation, but COL2 gene expression is believed to be suppressed by certain pro-inflammatory cytokines, such as IL-1β [22]. We found that deer bone extract recovered the mRNA expression of COL2 down-regulated by IL-1β (Fig. 2).
In chondrocytes, increases in MMP-1, -3, and -13 levels are related to cartilage degradation [2324]. MMPs, whose mRNA expressions are induced by pro-inflammatory cytokines, such as IL-1β, degrade the extracellular matrix (ECM) [25]. The MMPs are the principle proteins responsible for the degradation that occurs with normal remodeling of the ECM. They are a family of proteases that digest components of ECM and surface receptors [26]. IL-1β has the potential to induce cartilage degradation by up-regulating the expression of MMPs in cartilage [27]. In this study, we observed that deer bone extract could inhibit the up-regulation of MMPs in chondrocytes induced by IL-1β (Fig. 3, 4, and 5): As shown in Fig. 3, MMP-1 increase was inhibited by deer bone extract treatment, but there were no significant differences of MMP-1 expression in PC and DB from NC. Fig. 4 and 5 show that deer bone extract significantly recovered the mRNA expression of MMP-3, and -13 up-regulated by IL-1β.
Various nutritional ingredients have been investigated for OA treatment. These include chondroitin sulphate, which is plentiful in deer bone extract, as well as glucosamine, methyl-sulfonyl-methane, and S-adenosyl methionine. Chondroitin sulphate induces the production of proteoglycans and the IL-1β depletion of proteoglycan production in OA chondrocytes [2829]. Chondroitin sulphate is believed to provide building blocks for the synthesis of proteoglycans and increase sulphate incorporation in OA proteoglycans [3031]. Furthermore, decreasing the effects of MMPs by reducing their synthesis and activity could account for the beneficial effects of chondroitin sulphate treatment [32]. Chondroitin sulphate treatment in chondrocytes stimulates accumulation of molecules such as COL2 in the matrix, due in part to the down-regulation of MMPs. Chondroitin sulphate decreases the IL-1β-induced MMP gene expression in chondrocytes [33]. Therefore, chondroitin sulphate in deer bone extract could induce the accumulation of COL2 through the down-regulation of MMPs in IL-1β-induced OA chondrocytes. The disease modifying effect (the ability to retard progression of cartilage degeneration) of the mixture of the components related to OA is more efficacious than any component alone [34].
Glycosphingolipids (GSLs) are a group of glycolipids that are widely distributed on vertebrate plasma membranes. These molecules form clusters on cell membranes, where the GSLs modulate transmembrane signaling and mediate cell-to-cell and cell-to-matrix interactions [35]. GSLs are critical for the maintenance of chondrocyte homeostasis [36]. Gangliosides are abundant in mammalian cells. Deer bone extract contains 1.03 mg/g of ganglioside (Table 2). Among GSLs, gangliosides, including GM3 and its derivatives, are related to arthritic diseases [373839]. In the context of OA, the total ganglioside content of OA cartilage is decreased by 40% [3738].
The depletion of gangliosides enhanced OA development, elevating MMP-13 and a disintegrin and metalloproteinase with thrombospondin motifs-5 (ADAMTS-5) expression and accelerating chondrocyte apoptosis. Moreover, the overexpression of GM3-synthetase (GM3S) in vitro suppressed MMP-13 and ADAMTS-5 expression [40]. These results suggest that gangliosides suppress OA development and may provide a target for the design of novel anti-OA therapeutics.
Therefore, although chondroitin sulphate in deer bone extract is a major active component for OA treatment, we concluded that the collagen and ganglioside in deer bone extract related to OA may act synergistically in OA chondrocytes. In conclusion, we showed that treatment of OA chondrocytes with deer bone extract inhibited the IL-1β-induced gene expression of MMPs, classical markers of inflammation and cartilage degradation in arthritic joints, and resulted in the up-regulation of COL2. Thus, deer bone extract with various components related to OA, including chondroitin sulphate, possesses anti-osteoarthritic properties and can inhibit OA pathogenesis.
Figures and Tables
 | Fig. 1Effects of deer bone extract on the viability of chondrocytes.The points and bars represent means ± SD of triplicate experiments, respectively. Differences between treatments at each point were analysed using t-test and * means significantly (P < 0.05) different from the value for the cells without interleukin-1β (IL-1β) treatment. Each point indicates the percentage of viable chondrocytes compared to the initial number of viable chondrocytes.
|
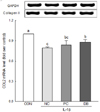 | Fig. 2Effects of deer bone extract on the mRNA expression of collagen type II (COL2) in interleukin-1β (IL-1β)-treated chondrocytes.Values are means ± SD of triplicate experiments. Differences between treatments were analysed using one way analysis of variance (ANOVA) by Tukey's multiple range tests. Means with different superscript letters are significantly different at P < 0.05. Treatments were assigned as follows: CON (PBS treatment); NC (IL-1β treatment); PC (IL-1β + 100 µg/mL glucosamine sulphate/chondroitin sulphate mixture); and DB (IL-1β + 100 µg/mL deer bone extract).
|
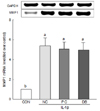 | Fig. 3Effects of deer bone extract on the mRNA expression of matrix metalloproteinase 1 (MMP-1) in interleukin-1β (IL-1β)-treated chondrocytes.Values are means ± SD of triplicate experiments. Differences between treatments were analysed using one way analysis of variance (ANOVA) by Tukey's multiple range tests. Means with different superscript letters are significantly different at P < 0.05. Treatments were assigned as follows: CON (PBS treatment); NC (IL-1β treatment); PC (IL-1β + 100 µg/mL glucosamine sulphate/chondroitin sulphate mixture); and DB (IL-1β + 100 µg/mL deer bone extract).
|
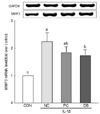 | Fig. 4Effects of deer bone extract on the mRNA expression of matrix metalloproteinase 3 (MMP-3) in interleukin-1β (IL-1β)-treated chondrocytes.Values are means ± SD of triplicate experiments. Differences between treatments were analysed using one way analysis of variance (ANOVA) by Tukey's multiple range tests. Means with different superscript letters are significantly different at P < 0.05. Treatments were assigned as follows: CON (PBS treatment); NC (IL-1β treatment); PC (IL-1β + 100 µg/mL glucosamine sulphate/chondroitin sulphate mixture); and DB (IL-1β + 100 µg/mL deer bone extract).
|
 | Fig. 5Effects of deer bone extract on the mRNA expression of matrix metalloproteinase 13 (MMP-13) in interleukin-1β (IL-1β)-treated chondrocytes.Values are means ± SD of triplicate experiments. Differences between treatments were analysed using one way analysis of variance (ANOVA) by Tukey's multiple range tests. Means with different superscript letters are significantly different at P < 0.05. Treatments were assigned as follows: CON (PBS treatment); NC (IL-1β treatment); PC (IL-1β + 100 µg/mL glucosamine sulphate/chondroitin sulphate mixture); and DB (IL-1β + 100 µg/mL deer bone extract).
|
References
1. Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin YE. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2005; 13:988–997.

2. Sanchez C, Deberg MA, Bellahcène A, Castronovo V, Msika P, Delcour JP, Crielaard JM, Henrotin YE. Phenotypic characterization of osteoblasts from the sclerotic zones of osteoarthritic subchondral bone. Arthritis Rheum. 2008; 58:442–455.

3. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013; 21:1145–1153.

4. Rashad S, Revell P, Hemingway A, Low F, Rainsford K, Walker F. Effect of non-steroidal anti-inflammatory drugs on the course of osteoarthritis. Lancet. 1989; 2:519–522.

5. Kean WF, Kean R, Buchanan WW. Osteoarthritis: symptoms, signs and source of pain. Inflammopharmacology. 2004; 12:3–31.

6. Leeb BF, Schweitzer H, Montag K, Smolen JS. A metaanalysis of chondroitin sulfate in the treatment of osteoarthritis. J Rheumatol. 2000; 27:205–211.
7. Pravin SK, Durgacharab AB, Dinesh MS. Deer antlers-traditional use and future perspectives. Indian J Tradit Knowl. 2010; 9:245–251.
9. Sunwoo HH, Nakano T, Sim JS. Isolation and characterization of proteoglycans from growing antlers of wapiti (Cervus elaphus). Comp Biochem Physiol B Biochem Mol Biol. 1998; 121:437–442.

10. Pham PH, Duffy TL, Dmytrash AL, Lien VW, Thomson AB, Clandinin MT. Estimate of dietary ganglioside intake in a group of healthy Edmontonians based on selected foods. J Food Compost Anal. 2011; 24:1032–1037.

11. Hara S, Yamaguchi M, Takemori Y, Furuhata K, Ogura H, Nakamura M. Determination of mono-O-acetylated N-acetylneuraminic acids in human and rat sera by fluorometric high-performance liquid chromatography. Anal Biochem. 1989; 179:162–166.

12. Salcedo J, Lacomba R, Alegria A, Barbera R, Matencio E, Lagarda MJ. Comparison of spectrophotometric and HPLC methods for determining sialic acid in infant formulas. Food Chem. 2011; 127:1905–1910.

13. Barbosa I, Garcia S, Barbier-Chassefière V, Caruelle JP, Martelly I, Papy-García D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology. 2003; 13:647–653.

14. Cheung HS, Harvey W, Benya PD, Nimni ME. New collagen markers of 'derepression' synthesized by rabbit articular chondrocytes in culture. Biochem Biophys Res Commun. 1976; 68:1371–1378.

15. Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988; 48:589–601.
16. Marlovits S, Hombauer M, Truppe M, Vècsei V, Schlegel W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J Bone Joint Surg Br. 2004; 86:286–295.

17. Lee SM, Moon J, Do HJ, Chung JH, Lee KH, Cha YJ, Shin MJ. Onion peel extract increases hepatic low-density lipoprotein receptor and ATP-binding cassette transporter A1 messenger RNA expressions in Sprague-Dawley rats fed a high-fat diet. Nutr Res. 2012; 32:210–217.

18. Sui Z, Zhang L, Huo Y, Zhang Y. Bioactive components of velvet antlers and their pharmacological properties. J Pharm Biomed Anal. 2014; 87:229–240.

19. Zhang L, Mu X, Fu J, Zhou Z. In vitro cytotoxicity assay with selected chemicals using human cells to predict target-organ toxicity of liver and kidney. Toxicol In Vitro. 2007; 21:734–740.

20. Yasuhara R, Miyamoto Y, Akaike T, Akuta T, Nakamura M, Takami M, Morimura N, Yasu K, Kamijo R. Interleukin-1β induces death in chondrocyte-like ATDC5 cells through mitochondrial dysfunction and energy depletion in a reactive nitrogen and oxygen species-dependent manner. Biochem J. 2005; 389:315–323.

21. Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, Poole AR. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994; 93:1722–1732.

22. Chandrasekhar S, Harvey AK, Higginbotham JD, Horton WE. Interleukin-1-induced suppression of type II collagen gene transcription involves DNA regulatory elements. Exp Cell Res. 1990; 191:105–114.

23. Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001; 44:585–594.

24. Wolfe GC, MacNaul KL, Buechel FF, McDonnell J, Hoerrner LA, Lark MW, Moore VL, Hutchinson NI. Differential in vivo expression of collagenase messenger RNA in synovium and cartilage. Quantitative comparison with stromelysin messenger RNA levels in human rheumatoid arthritis and osteoarthritis patients and in two animal models of acute inflammatory arthritis. Arthritis Rheum. 1993; 36:1540–1547.
25. Ma B, van Blitterswijk CA, Karperien M. A Wnt/beta-catenin negative feedback loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes. Arthritis Rheum. 2012; 64:2589–2600.

26. Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011; 3:a005058.

27. Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005; 52:128–135.

28. Nerucci F, Fioravanti A, Cicero MR, Collodel G, Marcolongo R. Effects of chondroitin sulfate and interleukin-1β on human chondrocyte cultures exposed to pressurization: a biochemical and morphological study. Osteoarthritis Cartilage. 2000; 8:279–287.

29. Wang L, Wang J, Almqvist KF, Veys EM, Verbruggen G. Influence of polysulphated polysaccharides and hydrocortisone on the extracellular matrix metabolism of human articular chondrocytes in vitro. Clin Exp Rheumatol. 2002; 20:669–676.
30. Schwartz NB. Regulation of chondroitin sulfate synthesis. Effect of beta-xylosides on synthesis of chondroitin sulfate proteoglycan, chondroitin sulfate chains, and core protein. J Biol Chem. 1977; 252:6316–6321.

31. Schwartz NB, Dorfman A. Stimulation of chondroitin sulfate proteoglycan production by chondrocytes in monolayer. Connect Tissue Res. 1975; 3:115–122.

32. Uebelhart D, Thonar EJ, Zhang J, Williams JM. Protective effect of exogenous chondroitin 4,6-sulfate in the acute degradation of articular cartilage in the rabbit. Osteoarthritis Cartilage. 1998; 6 Suppl A. 6–13.

33. Chan PS, Caron JP, Orth MW. Effect of glucosamine and chondroitin sulfate on regulation of gene expression of proteolytic enzymes and their inhibitors in interleukin-1-challenged bovine articular cartilage explants. Am J Vet Res. 2005; 66:1870–1876.

34. Lippiello L, Woodward J, Karpman R, Hammad TA. In vivo chondroprotection and metabolic synergy of glucosamine and chondroitin sulfate. Clin Orthop Relat Res. 2000; 229–240.

35. Hakomori SI. Structure and function of glycosphingolipids and sphingolipids: Recollections and future trends. Biochim Biophys Acta. 2008; 1780:325–346.

36. Seito N, Yamashita T, Tsukuda Y, Matsui Y, Urita A, Onodera T, Mizutani T, Haga H, Fujitani N, Shinohara Y, Minami A, Iwasaki N. Interruption of glycosphingolipid synthesis enhances osteoarthritis development in mice. Arthritis Rheum. 2012; 64:2579–2588.

37. David MJ, Hellio MP, Portoukalian J, Richard M, Caton J, Vignon E. Gangliosides from normal and osteoarthritic joints. J Rheumatol Suppl. 1995; 43:133–135.
38. David MJ, Portoukalian J, Rebbaa A, Vignon E, Carret JP, Richard M. Characterization of gangliosides from normal and osteoarthritic human articular cartilage. Arthritis Rheum. 1993; 36:938–942.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download