Abstract
BACKGROUND/OBJECTIVES
Turmeric (Curcuma longa L.) has been reported to have many biological functions including anti-obesity. Leptin, peptide hormone produced by adipocytes and its concentration is increased in proportion to the amount of the adipocytes. In the present study, we examined the effects of Korean turmeric on the regulation of adiposity and leptin levels in 3T3-L1 adipocytes and rats fed a high-fat and high-cholesterol diet.
MATERIALS/METHODS
Leptin secretion, free fatty acid and glycerol contents in 3T3-L1 adipocytes were measured after incubation of cells with turmeric for 24 hours. Rats were divided into four experimental groups: a normal diet group (N), a high-fat and high-cholesterol diet group (HF), a high-fat and high-cholesterol diet group supplemented with 2.5% turmeric extracts (TPA group) and a high-fat and high-cholesterol diet group supplemented with 5% turmeric extracts (TPB group). Serum samples were used for the measurement of leptin concentration.
RESULTS
Contents of free fatty acid and glycerol showed concentration dependent increase in response to turmeric extracts. Effects of turmeric extracts on reduction of lipid accumulation in 3T3-L1 cells were examined by Oil Red O staining. Treatment with turmeric extracts resulted in increased expression levels of adipose triglyceride lipase and hormone-sensitive lipase mRNA. The concentration of leptin from 3T3-L1 adipocytes was significantly decreased by turmeric. Proportional abdominal and epididymal fats weights of the turmeric 5% supplemented group, TPB has significantly decreased compared to the HF group. The serum levels of leptin in the TPA and TPB groups were significantly lower than those of the HF group.
Obesity is one of the most threatening modern disorders, raising the risk of serious metabolic diseases. Obesity is not simply an increase in weight, as weight can include muscle mass, but it is also defined by excessive accumulation of fat. Common wisdom holds that this results from an unbalance between energy intake, which enters the body through food, exceeding the energy that is consumed by exercising and metabolism [1]. This excessive accumulation of fat raises the risk of chronic diseases like diabetes mellitus, hypertension, and coronary artery disease [2]. Thus there is rising concern for the prevention and treatment of obesity. We can address the problems of obesity by controlling the absorption and storage of the nutritive substance. For direct control of fat, we can inhibit adipogenesis and lipogenesis and increase lipolysis [3].
Leptin, the product of the obesity gene, is an important hormone involved with feeding behavior, and its concentration in blood is increased in direct proportion to the amount of body fat [4]. When leptin is carried to the brain through the circulatory system, it is combined with leptin receptors of hypothalamic nerve cells that feeds the regulatory center and sends signals to the brain. This affects energy consumption, the metabolism of carbohydrate and fat, and body weight control [4]. Active research is examining the association of leptin and feeding behavior, hypothesizing that body weight control is related to obesity, and leptin is being used as the standard evaluating obesity [5]. Leptin is important in lipid metabolism as it controls the increase of fats and free fatty acids in blood [6]. In normal humans, circulating leptin is lowered due to decreased fat mass following weight reduction [78]. More leptin is secreted by obese people than by ordinary people [9], but leptin secreted continually by the excessive body fat is not effective, due to leptin resistance. Much remains to be learned about mechanisms underlying cellular leptin resistance and the significance of mediators of leptin resistance [1011].
Curcuma longa L., turmeric, a herbaceous perennial of the zingiberaceae, is distributed in South Asia, Africa, and Latin America including India, China, and Japan. Curcumin, a representative substance from the root of turmeric, includes about 2~8% turmeric and affects certain physiological functions [12]. Curcumin is consumed daily throughout Asian countries without reported toxicity [13]. Curcumin controls anti-inflammatory, anti-diabetes and immune regulation [12]. The effect of curcumin on anti-obesity and lipid metabolism is high [1214]. Shao et al. [15] observed that mice fed with a high-fat diet were able to control lipogenesis by curcumin. Ejae et al. [16] found that injection of curcumin into obese mice led to control of adipogenesis. An in vitro experiment using cells differentiated into adiposity from preadipocyte, 3T3-L1 from mouse embryo found that curcumin reduces leptin secretion [17]. These results show that curcumin contained in turmeric, contributes to controlling fat accumulation. Ling et al. [18] reported that the oil abstracted from the roots of turmeric controls lipid metabolism in blood. Dixit et al. [19] confirmed that ethanol extracts from turmeric are hypolipidemic in a rat model. However, evidence supporting the relation between the control of leptin secretion by turmeric and lipid metabolism, and research confirming control of leptin secretion in vivo are insufficient. Many recent studies report that Korean turmeric has the capacity to control lipid metabolism [2021].
Thus this study measures the effects of anti-obesity related to fat control using Korean turmeric. First, in vitro, we measured the concentration change of free fatty acids and glycerol produced by lipolysis caused by adding turmeric extracts. Expression levels of ATGL (adipose triglyceride lipase) and HSL (hormone-sensitive lipase) genes were investigated and secretion of leptin was examined from adipocytes stimulated by turmeric extracts. In vivo, after providing turmeric extracts for rats fed with high-fat and high-cholesterol diet, we assessed the effects of turmeric on the control of fat accumulation.
The turmeric was purchased from the Jindo-gun, Jeollanam-do, Korea. Air-dried turmeric was crushed using a grinder (Desung power mixer/grinder DA-280G, Seoul, Korea) and shattered turmerics were used in samples by cold storage. Grinded turmerics and six times distilled water (w/v) were mixed and then extraction was performed at 4℃ for 24 hours. This process was repeated three times and the supernatant was collected and filtered with a Whatman No.1 filter paper. The solvent of combined extracts was evaporated under reduced pressure using a rotary vacuum evaporator (HS-2001N, Hanshin Science Co., Korea) and dried in a freeze drier (Bondiro, Ilshin, Korea).
3T3-L1 preadipocytes were purchased from the ATCC (Americal Type Culture Collection, Manassans, VA, USA) and maintained in Dulbecco's Modified Eagle's Medium containing (DMEM)(Life Technologies, Grand Island, NY, USA) 10% fetal bovine serum and penicillin/streptomycin (100 IU and 100 µg/ml, respectively) at 37℃ in 5% CO2. Differentiation to the adipocyte form was induced by incubating 1 × 105 cells in DMEM containing 10% fetal bovine serum, 5 µg/ml insulin (Sigma Chemical, St. Louis, MO), 250 nM dexamethasone (Sigma Chemical), and 500 mM 3-isobutyl-1-methylxanthine (Sigma Chemical) for 3 days in 10 ml well cultured plates. Cells were then incubated for an additional 48 hours in DMEM containing 10% fetal bovine serum and 1 µg/ml insulin [22]. Subsequently, the medium was changed to stimulant-containing DMEM. Differentiated cells were stimulated by 2.5, 5 and 10 µg/ml of turmeric extracts for 24 hours. Free fatty acids were measured in the medium immediately before the cells were harvested. Free fatty acids were measured by commercial kit (SICDIA NFFAZYME, Shin Young Chemical IND, Korea), according to the manufacturer's instructions. 5 ul of medium containing 200 ul of NEFA reagent-1A was then reacted for 5 minutes, and measured an absorbance at 550 nm. Free fatty acid was calculated as the (sample absorbance/standard solution absorbance)×1000. 50 ul of medium containing 10 ul of glycerol reagent was then reacted for 1 minute into a new 96-well plate for glycerol measurement on a microplate reader at 570 nm. The amount of glycerol release was determined using method Bradford (Bio-rad, Hercules, CA) [23].
3T3-L1 cells were stimulated by 2.5, 5 and 10 µg/ml of turmeric extracts for 24 hours. After stimulating, cells were washed with phosphate buffered saline (PBS), fixed for 20 minutes with 4% formalin in PBS 0.05M. Cells were then stained with 0.35% Oil Red O solution (Sigma-Aldrich, Tokyo, Japan) in 60% isopropanol for 10 min at room temperature, washed 3 times with distilled water, and photographed.
Total RNA was extracted using Sepasol (Nacalai Tesque) with manufactural instructions. 2 µg of RNA were used for cDNA synthesis with ReverTra Ace (Toyobo, Tokyo, Japan). The sequences of primers (Invitrogen, Carlsbad, CA, USA) for analyzed genes were; 5'-ACCACAGTCCATGCCATCAC-3' and 5'-CACCACCCTGTTGCTGTAGCC-3' for GAPDH, 5'-GAGCCCCGGGGTGGAACAAGAT-3' and 5'-AAAAGGTGGTGGGCAGGAGTAA-3' for ATGL, 5'-CCTACTGCTGGGCTGTCAA-3' and 5'-CCATCTCGCACCCTCACT-3' for HSL. The PCR program was 30 cycles of denaturation at 98℃ for 10 seconds, annealing at 58-60℃ for 30 seconds and extension at 68℃ for 30 seconds by iCycler (Bio-Rad, Munich, Germany). The products were visualized by ethidium bromide agarose gels and the images were detected with UV irradiation. The densitometry result for target samples was divided by the densitometry result of GAPDH, a constitutive gene control, normalizing the level considered for analysis. Densitometry was carried out using a Bio-Rad ChemiDoc image acquisition system and Quantity One quantitation software (Bio-Rad, Hercules, CA, USA).
3T3-L1 cells were harvested 8 days after the initiation of differentiation. Subsequently, the medium was changed to stimulant-containing DMEM. Differentiated cells were stimulated by 2.5, 5 and 10 µg/ml of turmeric extracts for 24 hours. Subsequently, cells were washed twice with cold phosphate buffered saline and collected by scraping with a cell scraper into 50 mM Tris-HCl (pH 7.5) containing 1 mM EDTA. The harvested cells were sonicated for 5 seconds. After centrifugation at 13,000 g for 5 minutes at 48℃, the supernatants were assayed for leptin [22]. The concentration of leptin was measured by leptin immunoassay kit (Quantikine® M, R&D System, Minneapolis, MN), according to the manufacturer's instructions.
Cell viability was determined by using the MTT colorimetric assay [24]. This assay involves the ability of viable cells to convert a soluble tetrazolium salt, MTT{3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide}, into a blue formazan end product by mitochondrial dehydrogenase enzymes. Cells (1 × 105 cells per well) were plated in 96 well cultured plates, incubated for 24 hours, and treated with turmeric for 24 hours. MTT (2.5 mg/ml) was added to each well as incubation continued at 37℃ for an additional 4 hours. The media was then discarded and 100 µl of DMSO was added to each well to solubilise the coloured formazan product. Absorbance was read at 550 nm on a scanning microtiter spectrophotometer plate reader and was expressed as percentage of the absorbance obtained in the well treated with no stimulants [25].
Male Sprague-Dawley rats weighing 100 ± 10 g were purchased from Bio genomics (Seoul, Korea). Rats were individually housed in stainless steel cages in a room with controlled temperature (20-23℃) and lighting (alternating 12 h periods of light and dark). Rats were divided into four groups which were composed of normal diet group (N group), high-fat and high-cholesterol diet (HF group), high-fat and high-cholesterol diet and 2.5 g turmeric extracts/100 g diet group (TPA group) and high-fat and high-cholesterol diet and 5 g turmeric extracts/100 g diet group (TPB group) (Table 1). The rats fed the experimental diets for a period of 4 weeks. The experimental design was approved by the committee (NVRQS AEC5) of Korea International University regarding the care and use of laboratory animals. Serum samples were collected from the abdominal aorta and were centrifuged at 1,000 rpm for 10 minutes. The levels of serum leptin was measured by ELISA (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions.
Results were analyzed by ANOVA and Tukey's honestly significant difference test [26]. Differences were considered significant as at P < 0.05.
The cytotoxicity of the turmeric in 3T3-L1 cells was evaluated to determine the test concentration for the further analysis. The cell viability is shown in Fig. 1. The cell viability in the concentration of turmerics, 2.5, 5, 10, 20 and 40 µg/ml was 100, 97.72, 90.97, 34.94 and 16.77%, respectively. The cell viability assay showed that the turmerics affect no damage at concentrations of 2.5, 5 and 10 µg/ml. Therefore, we used the concentration of turmeric up to 10 µg/ml. Fig. 2A shows the level of free fatty acids, and one of the product of lipolysis. Lipolysis is the process in which triglycerides are hydrolyzed into free fatty acids and glycerol. Free fatty acid was significantly increased by 76.36, 93.18 and 171.82% compared with controls when 3T3-L1 cells were treated with the 2.5, 5 and 10 µg/ml of turmeric, respectively. Glycerol also was significantly increased by 64.55, 88.48 and 155.9% compared with controls (Fig. 2B). The results indicate that the turmeric has a function of lipolysis.
To investigate the effects of turmeric extracts on lipid decrease in differentiated 3T3-L1 cells, 3T3-L1 cells were treated with the 2.5, 5 and 10 µg/ml of turmeric extracts, respectively. After 24 hours, we found that turmeric extracts diminished the lipid accumulation in 3T3-L1 cells compared with that the untreated 3T3-L1 cells (Fig. 3).
To further define the lipolysis by turmeric extracts, ATGL and HSL of key enzymes involved in intracellular degradation of triglyceride are confirmed by gene expression (Fig. 4). Our results demonstrated as follows; mRNA levels of ATGL and HSL were increased in 3T3-L1 cells treated with turmeric extracts when compared to the untreated with the control group.
The result of contents of leptin as an indicator of lipid accumulation associated with lipolysis is shown in Fig. 5. Leptin level was significantly decreased by 11.86%, 24.68% and 30.27% compared with controls when 3T3-L1 cells were treated with the 2.5, 5 and 10 µg/ml of turmeric, respectively. These data suggest that turmeric can control leptin in adipocytes.
Proportional abdominal fat, epididymal fat and liver weights were measured, as shown in Table 2. The proportional abdominal and epididymal fats weights of the HF group were significantly greater than that of the N group. 5% turmeric extracts supplemented group, TPB was significantly decreased compared to the HF group. Especially, the TPB group was similar to that of the normal group. Accordingly, it is considered turmerics are effective in decreasing body fat. The proportional liver weight was no differences between the all of the experimental groups.
Fig. 6 shows change of leptin levels in the rats serum by turmeric. The result of leptin in the serum was greater in the HF, TPA and TPB groups than in the N group. The leptin in the TPA and TPB was 26.4% and 38.7% lower, respectively, than that in the HF group. Especially, TPB group showed the control of leptin secretion to the normal extent. These results indicate that turmeric is able to regulate secretion of leptin.
The increase and differentiation of adipocyte causes adipocyte to be excessively accumulated [27]. Thus for the prevention and treatment of obesity, the study on lipogenesis control mechanism is being made continually. Recently, new treatments which have an effect on the control of adipocyte are being developed, but natural substance of food becomes the center of public attention because of the various side effects of the treatment [2829].
The effect of lipid metabolism control of Indian turmeric having various biological active functions in is widely known so far [3031]. This study made an observation of the regulation of adipocyte related to anti-obesity using Korean turmeric which is being grown more and more recently. When triglyceride accumulation in adipocyte is hydrolyzed, it is divided into free fatty acids and glycerol in the body gets into blood outside cells and moves into many tissues and is used as the source of energy or stored as triglyceride in the liver and adipose tissue [32]. This can indicate the decomposition degree of triglyceride in adipocyte. And measuring the contents of free fatty acids and glycerol separated from 3T3-L1 adipocyte can be used as the measure indicating the decomposition degree of triglyceride in fat globule indirectly, so it is being used in many studies for the evaluation of lipolysis degree [33]. After adding turmeric extract in 2.5, 5 and 10 µg/ml to 3T3-L1 adipocyte, measuring free fatty acids and glycerol produced of lipolysis in medium, they tend to increase significantly (Fig. 2). This shows that the free fatty acids and glycerol hydrolyzed by turmeric are released and increased outside the cells. We found that turmeric extracts reduced lipid accumulation, as measured by Oil Red O staining (Fig. 3), and confirmed that increased mRNA levels of ATGL and HSL which are identified as an major triacylglycerol hydrolase by turmeric extracts (Fig. 4). ATGL predominantly performs the initial hydrolysis and provides diacylglycerol for subsequent reactions. HSL efficiently degrades diacylglycerol into monoacylglycerol and free fatty acid [34]. As a result, there is shown the high probability that turmeric extracts caused to decrease the lipid accumulation by triglyceride breakdown enzymes, ATGL and HSL. According to the study by Sengupta et al. [31], adding Indian turmeric extract in 10, 25 and 50 µg/ml to 3T3-L1 showed the effect controlling adipogenesis. Korean turmeric is thought to have a more powerful control on the lipid metabolism, because it can increase lipolysis in 2.5 and 5 µg/ml. Also, according to the study by Lee et al. [35] who used Korean turmeric, glycerol released into medium from 3T3-L1 adipocyte differentiated by methanol extract treatment of turmeric was increased. This suggests that Korean turmeric also has a great effect on the control of fat accumulation.
Leptin, peptide hormones secreted by obesity gene of adipocyte functions by combining with leptin receptors [36]. When leptin is secreted it functions in the hypothalamus that has to do with feeding control and the increase of energy consumption and is known as the protein keeping body fat to a certain extent and controlling obesity [36]. The increase of free fatty acids and glycerol caused by the decomposition of triglyceride by turmeric extracts treatment suggests the decrease of fat accumulation in adipocyte. Furthermore, we could confirm that turmeric extracts on reduction of lipid accumulation by Oil Red O staining. Thus we measured the concentration of leptin secreted in proportion to the increase of adipocyte and observed their correlation. When we added turmeric to 3T3-L1, adipocyte, the concentration of leptin decreased as that of turmeric increased (Fig. 5). We can say that the decrease of leptin secretion is related to the increase of lipolysis, because leptin is secreted from adipocyte. It was shown that leptin levels are tightly correlated with the total amount of white fat in the body [37]. It was also observed that curcumin inhibited adipocyte differentiation in 3T3-L1 adipocytes by intracellular lipid accumulation assay [38]. Thus it may be thought that the treatment of turmeric controls the differentiation of adipocyte and decreases the number and size of adipocyte. This also corresponded with the study by Ciardi et al. [17] that reported the secretion of leptin decreased by adding curcumin to 3T3-L1 adipocyte. In addition, this change of the concentration of leptin was confirmed in vivo. In the result of providing turmeric extracts for obese rats caused by a high-fat and high-cholesterol diet, leptin secretion increased by high-fat and high-cholesterol diet decreased and rats fed with 5% of turmeric extracts showed the control of leptin secretion to the normal extent (Fig. 6). The control of leptin secretion in vivo level by the supply of turmeric was first confirmed. We also confirmed that the proportional weights of abdominal and epididymal fats were reduced in 5% turmeric treated rats in a significant manner (Table 2). Leptin is a key body fat signaling molecules that are secreted by adipose tissues, and can directly regulate in vivo fat storage [39]. It is shown that the body fat reduction is closely associated with leptin secretion. Our previous research has shown that the body weight gain was reduced in turmeric extracts supplemented group [20]. According to the study by Kim et al. [40] that reported curcumin in turmeric controls the differentiation of adipocyte and we also have been demonstrated that Korean turmeric regulate lipid metabolism in serum and liver of rats fed with high-fats and high-cholesterol diets via UDP-glucuronyl transferase activity and control of bile acid [20]. This suggests that curcumin in turmeric is thought to contribute to the control of lipid metabolism effectively. The effect of lipolysis and leptin secretion control by Korean turmeric was known to correspond with the results of many studies made till now. Also the study Elahi et al. [30] using Indian turmeric reported that by providing 5% of turmeric extracts for obese rats caused by high-fat diets, there was the improvements in the effect of lipid metabolism in liver tissues and blood. In our previous study, we confirmed the effect of lipid metabolism by Korean turmeric using 2.5% of turmeric extracts [20]. In this study, we proved that providing 2.5 and 5% of Korean turmeric has to do with the control of leptin secretion (Fig. 6). Though it is necessary to compare the content of curcumin in Indian turmeric with that in Korean turmeric, as we can see from the previous results, we expect that Korean turmeric contains more curcumin substance related to lipid metabolism control than Indian turmeric.
Thus, this study proved that Korean turmeric can contribute to regulate leptin secretion related to lipid metabolism control. Turmeric may regulate energy metabolic process and decrease the quantity of body fat. Curcumin in turmeric reduces adipogenic differentiation by activation of Wnt/β-Catenin signaling [41]. Curcumin also directly interacts with adipocytes and immune system macrophages, thus assists in reducing leptin resistance, enhances adiponectin and lowers inflammatory indicators related with obesity, including leptin, resistin and MCP-1 [15]. Though more studies of the mechanism of triglyceride decomposition by turmeric and leptin are required, we may expect that Korean turmeric lowers body fats, and can be used as an anti-obesity agent by controlling fat.
Figures and Tables
 | Fig. 1Effects of turmeric extracts on viability of differentiating 3T3-L1 adipocytes.MTT colorimetric assay testing cell viability was performed after 24 hours of treatment with indicated concentration of turmeric. All values are presented as means ± SE. (n≥3). *Statistical significance determined by Tukey's test at P < 0.05.
|
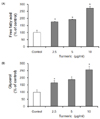 | Fig. 2Effects of turmeric extracts on free fatty acid (FFA) (A) and glycerol (B) of differentiating in 3T3-L1 adipocytes.FFA and glycerol assay was performed after 24 hours of treatment with indicated concentrations of turmeric extracts. Control indicates a sample stimulated only with medium. All values are presented as means ± SE. (n≥3). *Statistical significance determined by Tukey's test at P < 0.05. vs control.
|
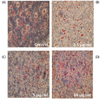 | Fig. 3Turmeric extracts inhibit lipid accumulation in 3T3-L1 adipocytes.Adipocytes were incubated with three concentrations (2.5, 5 and 10 µg/ml) of turmeric extracts and lipid accumulation was determined by Oil Red O staining and photographed. Lipid droplet formation in mature adipocytes (20X).
|
 | Fig. 4Expression of hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) mRNA from 3T3-L1 adipocytes stimulated by turmeric extracts.The effect of turmeric extracts on ATGL and HSL mRNA levels were investigated in 3T3-L1 adipocytes after 24 hours of treatment by RT-PCR analysis (A). Representative 2% agarose gel electrophoresis of PCR products stained with ethidium bromide. The right graphs show the mean band densitometry of ATGL or HSL/GAPDH relative expression.
|
 | Fig. 5Secretion of leptin from 3T3-L1 adipocytes in response to turmeric extracts.Leptin assay in 3T3-L1 adipocytes was performed after 24 hours of treatment with indicated concentrations of turmeric extracts. Control indicates a sample stimulated only with medium. All values are presented as means ± SE. (n≥3). *Statistical significance determined by Tukey's test at P < 0.05. vs control.
|
 | Fig. 6Effects of turmeric extracts on serum leptin of rats fed high-fat and high-cholesterol diets.The secretion of leptin from serum of rats fed normal diet (N), high-fat and high-cholesterol diet (HF) and high-fat and high-cholesterol diet with turmeric extracts 2.5% (TPA) or 5% (TPB) diets for 4 weeks. All values are the means ± SE (n = 10). Those with different superscript letters are significantly different at P < 0.05 by Tukey's test.
|
Table 2
Effects of turmeric extracts on change of proportional abdominal fat, epididymal fat and liver weights.

1)Proportional abdominal fat weight; (abdominal fat weight/body weight) × 100
2)Proportional abdominal fat weight; (epididymal fat weight/body weight) × 100
3)Proportional abdominal fat weight; (liver weight/body weight) × 100
4)All values are the means±SE (n=10).
5)NS: not significantly different among groups.
a-cThose with different superscript letters are significantly different at P < 0.05 by Tukey's test.
References
1. Ho JN, Jang JY, Yoon HG, Kim Y, Kim S, Jun W, Lee J. Anti-obesity effect of a standardised ethanol extract from Curcuma longa L. fermented with Aspergillus oryzae in ob/ob mice and primary mouse adipocytes. J Sci Food Agric. 2012; 92:1833–1840.

3. Lee M, Nam DE, Kim OK, Heo SH, Lee J. Lipolytic effect of supercritical extraction from Pine Cone (Pinus koraiensis) in mature 3T3-L1 adipocytes. J Korean Soc Food Sci Nutr. 2014; 43:1342–1348.

4. Homan P, Grob S, Milos G, Schnyder U, Eckert A, Lang U, Hasler G. The role of BDNF, leptin, and catecholamines in reward learning in bulimia nervosa. Int J Neuropsychopharmacol. 2014; 18:1–8.

5. Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995; 1:1155–1161.

6. Hennes MM, Dua A, Maas DL, Sonnenberg GE, Krakower GR, Kissebah AH. Relationships of plasma leptin levels to changes in plasma free fatty acids in women who are lean and women who are abdominally obese. Obes Res. 1997; 5:442–446.

7. Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003; 111:1409–1421.

8. Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004; 351:987–997.

9. Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996; 81:4406–4413.

10. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998; 395:763–770.

11. Myers MG Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010; 21:643–651.

13. Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991; 57:1–7.
14. Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008; 149:3549–3558.

15. Shao W, Yu Z, Chiang Y, Yang Y, Chai T, Foltz W, Lu H, Fantus IG, Jin T. Curcumin prevents high-fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One. 2012; 7:e28784.
16. Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. 2009; 139:919–925.

17. Ciardi C, Jenny M, Tschoner A, Ueberall F, Patsch J, Pedrini M, Ebenbichler C, Fuchs D. Food additives such as sodium sulphite, sodium benzoate and curcumin inhibit leptin release in lipopolysaccharide-treated murine adipocytes in vitro. Br J Nutr. 2012; 107:826–833.

18. Ling J, Wei B, Lv G, Ji H, Li S. Anti-hyperlipidaemic and antioxidant effects of turmeric oil in hyperlipidaemic rats. Food Chem. 2012; 130:229–235.

19. Dixit VP, Jain P, Joshi SC. Hypolipidaemic effects of Curcuma longa L and Nardostachys jatamansi, DC in triton-induced hyperlipidaemic rats. Indian J Physiol Pharmacol. 1988; 32:299–304.
20. Kim MS, Chun SS, Kim SH, Choi JH. Effect of tumeric (Curcuma longa) on bile acid and UDP-glucuronyl transferase activity in rats fed a high-fat and -cholesterol diet. J Life Sci. 2012; 22:1064–1070.

21. Kim TH, Son YK, Hwang KH, Kim MH. Effects of Angelica keiskei Koidzumi and turmeric extract supplementation on serum lipid parameters in hypercholesterolemic diet or P-407-induced hyperlipidemic rats. J Korean Soc Food Sci Nutr. 2008; 37:708–713.

22. Hong JH, Hwang EY, Kim HJ, Jeong YJ, Lee IS. Artemisia capillaris inhibits lipid accumulation in 3T3-L1 adipocytes and obesity in C57BL/6J mice fed a high fat diet. J Med Food. 2009; 12:736–745.

23. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254.

24. Carmichael J, DEGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 1987; 47:943–946.
25. Navarro-Escobar E, González-Rodríguez MP, Ferrer-Luque CM. Cytotoxic effects of two acid solutions and 2.5% sodium hypochlorite used in endodontic therapy. Med Oral Patol Oral Cir Bucal. 2010; 15:e90–e94.

26. Sreel RG, Torrie JH. Principles and Procedures of Statistics. New York: Mcgrow Hill;1980.
28. Jang YS, Jeong JM. Effects of phyto-extract mixture on adiposity and serum lipid levels in obese mice induced by high fat diet. J Korean Soc Food Sci Nutr. 2010; 39:1439–1445.

29. Apfelbaum M, Vague P, Ziegler O. IIanotin C, Thomas F, Leutenegger E. Long-term maintenance of weight loss after a very low calorie diet: efficacy and tolerability of sibutramine. Am J Med. 1999; 106:179–184.

30. Elahi RK. Preventive effects of turmeric (Curcuma longa Linn.) powder on hepatic steatosis in the rats fed with high fat diet. Life Sci J. 2012; 9:5462–5468.
31. Sengupta K, Golakoti T, Chirravuri VR, Marasetti AK. An herbal formula LI85008F inhibits lipogenesis in 3T3-L1 adipocytes. Food Nutr Sci. 2011; 2:809–817.

32. Frayn KN, Coppack SW, Fielding BA, Humphreys SM. Coordinated regulation of hormone-sensitive lipase and lipoprotein lipase in human adipose tissue in vivo: implications for the control of fat storage and fat mobilization. Adv Enzyme Regul. 1995; 35:163–178.

33. Hsu CL, Yen GC. Phenolic compounds: evidence for inhibitory effects against obesity and their underlying molecular signaling mechanisms. Mol Nutr Food Res. 2008; 52:53–61.

34. Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004; 306:1383–1386.

35. Lee J, Jun W. Methanolic extract of turmeric (Curcuma longa L.) enhanced the lipolysis by up-regulation of lipase mRNA expression in differentiated 3T3-L1 adipocytes. Food Sci Biotechnol. 2009; 18:1500–1504.
36. Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV. Leptin: the tale of an obesity gene. Diabetes. 1996; 45:1455–1462.

38. Kim CY, Le TT, Chen C, Cheng JX, Kim KH. Curcumin inhibits adipocyte differentiation through modulation of mitotic clonal expansion. J Nutr Biochem. 2011; 22:910–920.

39. Burdakov D, González JA. Physiological functions of glucose-inhibited neurones. Acta Physiol (Oxf). 2009; 195:71–78.

40. Kim J, Park J, Jun W. Anti-obesity effect of ethyl acetate fraction from 50% ethanol extract of fermented Curcuma longa L. in 3T3-L1 cells. J Korean Soc Food Sci Nutr. 2014; 43:1681–1687.

41. Ahn J, Lee H, Kim S, Ha T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am J Physiol Cell Physiol. 2010; 298:C1510–C1516.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download