Abstract
BACKGROUND/OBJECTIVES
The accumulation of amyloid-β (Aβ) in the brain is a hallmark of Alzheimer's disease (AD) and plays a key role in cognitive dysfunction. Perilla frutescens var. japonica extract (PFE) and its major compound, rosmarinic acid (RA), have shown antioxidant and anti-inflammatory activities. We investigated whether administration of PFE and RA contributes to cognitive improvement in an Aβ25-35-injected mouse model.
MATERIALS/METHODS
Male ICR mice were intracerebroventricularly injected with aggregated Aβ25-35 to induce AD. Aβ25-35-injected mice were fed PFE (50 mg/kg/day) or RA (0.25 mg/kg/day) for 14 days and examined for learning and memory ability through the T-maze, object recognition, and Morris water maze test.
RESULTS
Our present study demonstrated that PFE and RA administration significantly enhanced cognition function and object discrimination, which were impaired by Aβ25-35, in the T-maze and object recognition tests, respectively. In addition, oral administration of PFE and RA decreased the time to reach the platform and increased the number of crossings over the removed platform when compared with the Aβ25-35-induced control group in the Morris water maze test. Furthermore, PFE and RA significantly decreased the levels of nitric oxide (NO) and malondialdehyde (MDA) in the brain, kidney, and liver. In particular, PFE markedly attenuated oxidative stress by inhibiting production of NO and MDA in the Aβ25-35-injected mouse brain.
Alzheimer's disease (AD), a common neurodegenerative disorder of the elderly, is related to senile plaques and neurofibrillary tangles in the brain [1]. The principal component of senile plaques is amyloid-β (Aβ) protein, which is produced by amyloid precursor protein. Deposition of Aβ plaques affects synaptic function and is associated with learning and memory impairment in the human and mouse brain [23]. Memory loss and cognitive dysfunction are the main symptoms of AD patients. Therefore, an Aβ-induced AD model is used to search for novel agents with protective effects against neurotoxicity and AD. Oxidative stress caused by overproduction of free radicals plays a key role in AD and Aβ neurotoxicity [4]. In addition, Aβ increases inflammation and oxidative stress in in vivo models used to study AD [5]. Oxidative stress is the key event in the AD brain, and overproduction of reactive oxygen species (ROS) is related to mitochondrial dysfunction, which causes neurodegeneration [6]. Injection of pre-aggregated Aβ25-35 peptide is used to investigate molecular, morphological, and behavioral effects in non-transgenic in vivo models [7]. Therefore, we used an Aβ25-35-induced AD mouse model to investigate the effects of Perilla frutescens var. japonica extract (PFE) and rosmarinic acid (RA) on AD.
P. frutescens is an annual herbaceous plant used in Asian countries including Korea and Japan. P. frutescens leaves are used as fresh vegetables for wrapping meats, sedatives, and to treat food poisoning [89]. Kim et al. [10] demonstrated that PFE provided hepatic protection against oxidative damage. RA, a major polyphenol compound, has been reported to be an antioxidant and anti-inflammatory agent [1112]. These reports suggest that RA plays a crucial role in preventing brain-related oxidative stress and neurotoxicity. However, there have been no in vivo investigations of the protective effects of RA and PFE on learning and memory dysfunctions induced by Aβ neurotoxicity. Therefore, we used an Aβ25-35-injected mice model to assess the protective effects of RA and PFE on learning and memory impairment.
PFE was collected from Miryang, from August to September of 2008. Specimens were freeze-dried (SFDSM06, Samwon Co., Pusan, Korea) and extracted three times with 20 volumes of 100% methanol at room temperature for 24 h. The extract was then obtained using a rotary evaporator and the yield was 23.43%. RA, one of the major constituents of PFE [13], was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The methanol extracts from PFE and RA were dissolved in phosphate-buffered saline and dimethylsulfoxide (DMSO), respectively.
Aβ25-35 and malondialdehyde (MDA) were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). DMSO and NaCl were purchased from Bio Basics Inc. (Ontario, Canada). Thiobarbituric acid (TBA) was obtained from Lancaster Synthesis (Ward Hill, MA, USA). Phosphoric acid and butanol were acquired from Samchun Pure Chemical Company (Gyeonggi, Pyeongtaek, Korea).
Male 5-week-old ICR mice (Orient Inc., Seongnam, Korea) weighing 25-27 g were housed in plastic cages with free access to food and water and maintained in a controlled environment (20 ± 2℃, 50 ± 10% relative humidity, 12-h light/dark cycle). Mice were divided into four groups comprising eight individuals in each of four cages. The groups were defined as follows: normal group, which received 0.9% NaCl injection plus oral administration of water; control group, which received Aβ25-35 injection plus oral administration of water; PFE group, which received Aβ25-35 injection plus oral administration of PFE (50 mg/kg/day); and RA group, which received Aβ25-35 injection plus oral administration of RA (0.25 mg/kg/day) for 14 days using a sonde. There were no significant differences in initial body weight among groups. Animal experiments were conducted according to the Guidelines for Care and Use of Laboratory Animals published by the Pusan National University Institutional Animal Care and Use Committee (IACUC, approval number PNU-2009-0042).
Aβ25-35 was aggregated according to the procedure outlined by Maurice et al. [14]. In brief, the peptide was dissolved and diluted in 0.9% NaCl to achieve a concentration of 5 nmol. Aβ25-35 was incubated at 37℃ for 3 days before injection to induce aggregation. Aggregated Aβ25-35 was injected into mice according to the procedure established by Laursen and Belknap [15]. Mice were lightly anesthetized with CO2, after which the solution was injected 0.8 mm posterior to the bregma and 1.5 mm lateral to the sagittal suture. All injections were made using a 10 µl Hamilton microsyringe fitted with a 26 gauge needle that was inserted 2.2 mm beneath the surface of the brain. Animals were injected with 5 µl of 0.9% NaCl or 5 nmol Aβ25-35 aggregate in each cerebral lateral ventricle at a rate of 1 µl/min and the needle was left in the injection site for 1 min. The behavioral experimental schedule of mice injected with Aβ25-35 is shown in Fig. 1.
The T-maze test was conducted according to the procedure established by Montgomery [16]. The maze apparatus was T-shaped, and the walls were made of black boards (length of start and goal stems = 50 cm, width = 13 cm, height = 20 cm) that were glued to a square black board bottom. The maze consisted of a start box, left arm, and right arm with a door to separate the two sides. The mice were placed at the start box, and the number of touches and exploration times of the right arm of the T-maze were recorded during a 10-min period (training session). The mice were then placed back into the same apparatus 24 h after the training session. Animals were allowed to explore the right and left sides of the maze freely for 10 min, and the number of touches and exploration times were recorded (test session). Space perceptive ability (percent) was calculated as the ratio of the number of left or right maze entries to the number of total maze entries multiplied by 100.
The object recognition test was performed in a square black open-field apparatus (40 × 30 × 20 cm) [17]. Two identical objects (plastic bottles) were placed at fixed distances within the square field. The mice were then placed at the center of the square field, and the number of touches of each object was recorded during a 10 min period (training session). Next, the mice were placed back into the same field 24 h after the training session, but one of the objects used during the training session was replaced with a new object (another plastic bottle). The mice were allowed to explore freely for 10 min, and the number of touches was recorded (test session). Object cognitive ability (percent), a ratio of the amount of time spent exploring any one of the two original objects (training session) or the novel object (test session) over the total time spent exploring both objects was used to measure cognitive function.
The Morris water maze test was conducted according to the procedure established by Morris, with slight modifications [18]. The apparatus used in the Morris water maze test consisted of a dark plastic circular pool, 80 cm in diameter, surrounded by a 40-cm-high wall randomly divided into quadrants. White poster color was added to the pool water to make it opaque, and the water temperature was maintained at 22 ± 1℃. A platform 8 cm in diameter was placed 1 cm below the water surface in the middle of one quadrant. The position of the platform was unchanged during the training session. Four posters on the walls of the apparatus provided visual cues for navigation. Three training trials per day were conducted for 3 days. During the training trials, mice were placed at random in the water facing the pool wall and allowed to swim for a maximum of 60 s. The latency time required to find the platform was recorded. Mice that found the platform were allowed to rest there for 15 s. If they failed to find the platform within 60 s, they were allowed to stay on the platform for 15 s to help them remember. A probe trial of the Morris water maze test was performed 1 day after the completion of training. In the primary test, the experiment was performed as before. In the secondary test, the trial was performed without the platform. The mice were placed in the pool and swam for 60 s while looking for the platform, and the latency time that the mice spent in the position previously occupied by the platform was recorded. In the tertiary test, the water was transparent and the number of trials that it took the mouse to reach the platform, which was visible 1 cm above the surface of the water, was counted. Occupancy of the target quadrant was calculated as the percentage of time spent in the target quadrant during a 60 s trial.
MDA levels were measured by the method described by Ohkawa et al. [19]. After completion of the behavioral observations, mice were anesthetized with CO2 and their brain, liver, and kidneys were removed immediately and placed on ice. The dissected tissue was then homogenized with saline solution and mixed with 1% phosphoric acid and 0.67% TBA solution. Following boiling for 45 min, the solution mixture was cooled in an ice bath, after which 2 ml of butanol was added and the samples were centrifuged at 3,000 rpm for 10 min. The absorbance values of the supernatant were measured at 535 and 520 nm and the level of lipid peroxidation was calculated using a MDA standard curve.
The nitric oxide (NO) concentration in tissues was determined as described by Schmidt et al. [20]. Briefly, 150 µl of supernatant from the lipid peroxidation procedure was mixed with 130 µl of distilled water, after which 20 µl of the dilution was added to the same amount of phosphoric acid and 0.1% n-(1-naphthyl) ethylenediamine dihydrochloride. The absorbance value of the mixture was measured at 540 nm and the NO yield was calculated using a sodium nitrite (NaNO2) standard curve.
The effects of PFE and RA on short-term memory were investigated using a T-maze test and measured by the number of entries into the different arms (Fig. 2). The results revealed that mice in the normal group showed a significant preference for the new arm, whereas those in the Aβ25-35-treated control group did not show differences in the number of arm entries between old and new arms. However, mice in the PFE and RA groups displayed a significantly higher number of entries in the new arm. These results suggested that PFE and RA improve spatial learning memory against Aβ25-35.
The effects of PFE and RA on Aβ25-35-induced memory impairment were investigated using a novel object recognition test (Fig. 3). The same two objects were explored during the training session. After 24 h, one of the familiar objects was replaced with a novel object. The control group showed no significant difference between recognition of the familiar and novel objects. However, groups administered PFE and RA touched the novel object more times than the familiar object, and mice treated with PFE (50 mg/kg/day) had the highest novel object cognition ability. According to our results, mice orally administered PFE and RA showed higher recognition ability toward novel objects in the Aβ25-35-induced cognitive impairment model.
To investigate whether PFE and RA improved the memory deficit induced by Aβ25-35, the Morris water maze test was conducted. As shown in Fig. 4, all groups showed decreased time to reach the platform during the training session. After 3 days of training, the PFE and RA groups showed significantly decreased time to reach the platform relative to that of the Aβ25-35-injected control group. On the final day, the hidden platform was removed and the time spent in the target quadrant was measured. The Aβ25-35-injected control group showed low occupancy of the target quadrant compared with the normal group. However, the PFE and RA groups increased their occupancy of the target quadrant (46.1% and 51.7%, respectively), as shown in Fig. 5. Conversely, in the visible platform test, no significant differences in latency to reach the platform were observed among groups, indicating that visual and exercise ability did not affect the cognitive and memory function in the Aβ25-35-induced AD model (Fig. 6).
Table 1 shows the inhibitory effects of PFE and RA against lipid peroxidation induced by Aβ25-35. Our results indicated that injection of Aβ25-35 significantly increased MDA levels in the brain, liver, and kidney. However, the PFE and RA administration groups showed reduced MDA levels in the kidney relative to the Aβ25-35-induced control group. Furthermore, the liver MDA levels of the PFE group were noticeably lower than those of the control group. In particular, oral administration of PFE and RA markedly attenuated MDA levels in the brain by 21.07% and 51.04%, respectively, when compared with the Aβ25-35-injected control group. These results indicate that PFE and RA inhibited MDA generation in the brain, liver, and kidney.
The NO scavenging effect of PFE and RA in the brain, liver, and kidney is shown in Table 2. When compared with the normal group, there was a significant increase in NO levels in the brains of Aβ25-35-injected mice. However, PFE and RA administration resulted in decreased NO levels. Specifically, PFE at a dose of 50 mg/kg/day significantly decreased NO levels in the brain relative to the control group. Moreover, the NO levels in the livers decreased significantly in response to treatment with PFE and RA when compared with the Aβ25-35-induced control group. These findings suggested that PFE and RA treatment attenuated the NO production induced by Aβ25-35 in these tissues.
Deposition of Aβ in the brain is the pathological hallmark of AD, and aggregation of Aβ induces neurotoxicity in neuronal cells and in vivo [2122]. The AD brain is subject to oxidative stress, as evidenced by lipid, protein, DNA oxidation and various other aspects [23]. Many previous studies have reported that natural sources prevent cognitive impairment and help improve memory function in elderly people by attenuating oxidative stress and Aβ deposition. Thus, administration of natural antioxidants is one method of preventing the brain damage in AD induced by oxidative stress [2425].
PFE is commonly used as a traditional medicine to treat food poisoning in Asia [26]. Other reports have shown that PFE might be useful for inhibiting inflammatory responses and scavenging ROS [27]. PFE is known to contain various active compounds such as RA, catechin, apigenin, luteolin, caffeic acid and ferulic acid. Among these, RA is one of the most prominent constituents of PFE [2829]. Ono et al. [30] suggested that RA inhibited fibril formation from Aβ in vitro. In addition, RA inhibited ROS formation and lipid peroxidation, suggesting a protective role on oxidative stress. Accumulating evidence suggests that RA from PFE plays a protective role against AD via regulation of oxidative stress in the brain. However, the effects of PFE and RA on cognitive function and learning and memory ability have not yet been evaluated in vivo. The present study was conducted to investigate the protective effects of PFE and RA against Aβ25-35-induced AD. Specifically, we investigated whether oral supplementation of PFE and its active compound, RA, could improve cognitive ability using T-maze, object recognition, and the Morris water maze in the AD mouse model. Furthermore, inhibitory effects against lipid peroxidation and NO production were also measured.
A previous study showed that PFE has beneficial effects in renal disease or failure in vivo by oral administration at 50 mg/kg per day [31]. In addition, RA at a dose of 0.25 mg/kg significantly prevented the memory impairment induced by ONOO- [32]. Furthermore, no toxicity was observed at doses up to 3000 mg/kg [10]. Based on these studies, we administered PFE and RA at 50 mg/kg and 0.25 mg/kg, respectively.
To evaluate spatial memory, we conducted a T-maze test in Aβ25-35-injected mice. In the Aβ25-35-injected control group, cognitive ability did not differ significantly between the old and new routes, indicating that Aβ25-35 induced cognitive impairment. However, the PFE and RA groups showed a high rate of exploring the new route. Our results are in agreement with those of another study showing that RA was effective in a plus-maze test, and that it suppressed neurotoxic properties [33]. These data indicate that PFE and RA were able to improve spatial memory and learning performances against Aβ25-35-induced cognitive dysfunction.
We examined the effects of PFE and RA administration in an object recognition test in Aβ25-35-injected mice. If the mice remember the previous familiar object, they spend more time exploring the novel object [34]. After a training trial, normal mice in the test trial touched the novel object while exploring more times than the familiar object. However, the Aβ25-35-treated control group showed no significant difference in the number of touches between the familiar and novel objects because they did not remember the familiar object. The PFE and RA groups showed cognitive recognition of the new object. In particular, the PFE group had the highest index in novel object awareness among groups.
The effects of PFE and RA on long-term memory were investigated in the Morris water maze. In the training session, the latency in the PFE and RA groups when compared with that of the Aβ25-35-treated control group was significantly shortened by repeating the study for 3 days. These results suggest that the learning ability of mice was disturbed by injection of Aβ25-35. Furthermore, PFE and RA groups located the platform by swimming across the pool and remembered the quadrant after the platform was removed. In contrast, the Aβ25-35-induced control group did not reduce the latency time to reach the platform. However, the time to reach the exposed platform did not differ significantly among groups. Consequently, these results suggest that PFE and RA protect against long-term memory impairment induced by Aβ25-35.
Working memory is a multi-component system served by a number of distinct neural circuits. Accumulation of Aβ in the AD brain, which may lead to cognitive decline, is associated with progressive disruption of neural circuits in the cortical and hippocampal network [3536]. Hippocampus-dependent spatial memory and hippocampus-independent non-spatial memory is affected by Aβ25-35. Flavonoids can have beneficial effects such as reducing neuroplastic changes in neural circuits [37]. Our results showed that the PFE and RA administered group performed better in spatial and non-spatial memory during the T-maze and novel object recognition tests, respectively. These findings suggest that PFE and RA exert a protective role against disruption of neural circuits in AD.
Because of the high concentration of polyunsaturated fatty acids and high oxygen consumption, the brain is especially vulnerable to oxidative stress [38]. Aβ is associated with ROS production, leading to mitochondrial dysfunction, lipid peroxidation, and cell death [39]. Thiobarbituric acid-reactive substance (TBARS) levels increased in all regions of AD brain, suggesting that TBARS is an indicator of oxidative stress in AD [40]. MDA, a lipid peroxidation biomarker, induces neuronal damage in AD [41]. Aβ is produced by many different cell types and circulates in blood and cerebrospinal fluid [42]. Oxidative stress induced by Aβ leads to lipid peroxidation in the AD brain and peripheral tissues or circulating cells [43]. Our previous studies also demonstrated that the injection of mice with Aβ25-35 led to elevated oxidative stress and lipid peroxidation in the kidney, liver and brain [444546]. In the present study, Aβ25-35-induced oxidative stress was shown in various tissues and cells. However, our results demonstrated that PFE and RA have protective effect against oxidative stress induced by Aβ25-35 in the liver and kidney, as well as in the brain. Schirrmacher et al. [47] observed that consumption of PFE influences antioxidant and lipid peroxidation in a beneficial manner. Their results showed that volunteers who consumed perilla had decreased plasma MDA concentrations. Another previous study also indicated that RA could reduce ROS formation and lipid peroxidation against Aβ in neuronal cells [48]. This evidence suggests that RA could play a neuroprotective role against oxidative damage.
NO derived from nitric oxide synthase plays an important role in neural signaling and immunomodulation. There is much evidence that Aβ stimulation can result in excessive production of NO, which is converted to peroxynitrite and induces oxidative damage [49]. Therefore, the scavenging of NO using natural ingredients from food intake could be necessary for prevention of AD. Our data demonstrated that NO levels increased in tissues after Aβ25-35 injection in mice. However, PFE and RA significantly prevented the Aβ25-35-induced NO generation, especially in the brain. Therefore, we assume that PFE and RA protect against oxidative stress through inhibition of the production of MDA and NO.
The blood-brain barrier (BBB) is a tightly sealed barrier between circulating blood and the central nervous system (CNS). The BBB is essential to the normal function of CNS and neuronal survival [50]. In AD pathology, the BBB becomes compromised, which affects Aβ production. Aβ exerts a toxic effect on brain vessels that results in impairment of BBB function [51]. In addition, the release of neurotoxic factors and ROS can lead to dysfunction of the BBB, which could further potentiate neuroinflammatory responses by allowing peripheral inflammatory cells, thus creating a vicious cycle [52]. According to Falé et al. [53], when RA was administered to rats it was detected in the plasma and brain. These findings indicate that RA can probably cross the BBB, reaching the rat brain. Based on these results, the regulatory role of BBB permeability by RA can likely be attributed to the protective role in Aβ25-35-induced memory impairment.
Plant extracts and active compounds are attracting a great deal of attention owing to their potential to improve cognitive function [54]. In conclusion, administration of PFE and RA improve learning and memory deficits in Aβ25-35-induced mice in T-maze, object recognition, and Morris water maze tests. In addition, the protective effect of PFE and RA against oxidative stress was related to the inhibition of NO and MDA levels. These results indicate that PFE and RA could be good sources for suppressing cognitive impairment and improving memory and learning ability. We expect PFE and RA to be useful to preventing and delaying the progression of memory impairment observed in AD.
Figures and Tables
 | Fig. 2Effects of Perilla frutescens var. japonica extract (PFE) and rosmarinic acid (RA) on spontaneous alternation behavior in the T-maze test.Data represent the means ± SD. (n = 8) * P < 0.05 compared with old route. PFE: Perilla frutescens var. japonica extract administered orally at 50 mg/kg/day. RA: rosmarinic acid administered orally at 0.25 mg/kg/day.
|
 | Fig. 3Effects of Perilla frutescens var. japonica extract (PFE) and rosmarinic acid (RA) on recognition memory in the novel object recognition test.Data represent the means ± SD. (n = 8) * P < 0.05 compared with familiar object. PFE: Perilla frutescens var. japonica extract administered orally at 50 mg/kg/day; RA: rosmarinic acid administered orally at 0.25 mg/kg/day.
|
 | Fig. 4Effect of Perilla frutescens var. japonica extract (PFE) and rosmarinic acid (RA) on escape latency to platform in the Morris water maze test.Data represent means ± SD. (n = 8) * P < 0.05 compared with normal group, # P < 0.05 compared with Aβ25-35-treated control group. PFE: Perilla frutescens var. japonica extract administered orally at 50 mg/kg/day; RA: rosmarinic acid administered orally at 0.25 mg/kg/day.
|
 | Fig. 5Effect of Perilla frutescens var. japonica extract (PFE) and rosmarinic acid (RA) on occupancy time in the target quadrant in the Morris water maze test.The percentage of time spent in the target quadrant was calculated in the water maze test on the final test day. Data represent means ± SD. (n = 8) * P < 0.05 compared with normal group, # P < 0.05 compared with Aβ25-35-treated control group. PFE: Perilla frutescens var. japonica extract administered orally at 50 mg/kg/day; RA: rosmarinic acid administered orally at 0.25 mg/kg/day.
|
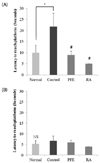 | Fig. 6Effect of Perilla frutescens var. japonica extract (PFE) and rosmarinic acid (RA) on latency to reach hidden platform (A) and exposed platform (B) in the Morris water maze test.Data represent the means ± SD. (n = 8) * P < 0.05 compared with normal group, # P < 0.05 compared with Aβ25-35-treated control group. NS indicates no significant differences among experimental groups. PFE: Perilla frutescens var. japonica extract administered orally at 50 mg/kg/day; RA: rosmarinic acid administered orally at 0.25 mg/kg/day.
|
Table 1
Protective activity of Perilla frutescens var. japonica extract (PFE) and rosmarinic acid (RA) against lipid peroxidation in mice injected with Aβ25-35

Notes
This work was carried out with the support of the Cooperative Research Program for Agriculture Science & Technology Development (PJ01015603), Rural Development Administration, Republic of Korea. This research was also supported by Global PH.D Fellowship Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015H1A2A1032531).
References
1. Collerton D. Cholinergic function and intellectual decline in Alzheimer's disease. Neuroscience. 1986; 19:1–28.

2. Golde TE, Eckman CB, Younkin SG. Biochemical detection of Aβ isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer's disease. Biochim Biophys Acta. 2000; 1502:172–187.

3. Sambamurti K, Greig NH, Lahiri DK. Advances in the cellular and molecular biology of the beta-amyloid protein in Alzheimer's disease. Neuromolecular Med. 2002; 1:1–31.
4. Harman D. Free radical theory of aging: Alzheimer's disease pathogenesis. Age (Omaha). 1995; 18:97–119.

5. Götz J, Ittner LM. Animal models of Alzheimer's disease and frontotemporal dementia. Nat Rev Neurosci. 2008; 9:532–544.

6. Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS. ABAD enhances Aβ-induced cell stress via mitochondrial dysfunction. FASEB J. 2005; 19:597–598.
7. Zussy C, Brureau A, Delair B, Marchal S, Keller E, Ixart G, Naert G, Meunier J, Chevallier N, Maurice T, Givalois L. Time-course and regional analyses of the physiopathological changes induced after cerebral injection of an amyloid β fragment in rats. Am J Pathol. 2011; 179:315–334.

8. Honda G, Koezuka Y, Kamisako W, Tabata M. Isolation of sedative principles from Perilla frutescens. Chem Pharm Bull (Tokyo). 1986; 34:1672–1677.

9. Kurita N, Koike S. Synergistic antimicrobial effect of sodium chloride and essential oil components. Agric Biol Chem. 1982; 46:159–165.

10. Kim MK, Lee HS, Kim EJ, Won NH, Chi YM, Kim BC, Lee KW. Protective effect of aqueous extract of Perilla frutescens on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem Toxicol. 2007; 45:1738–1744.

11. Gao LP, Wei HL, Zhao HS, Xiao SY, Zheng RL. Antiapoptotic and antioxidant effects of rosmarinic acid in astrocytes. Pharmazie. 2005; 60:62–65.
12. Takano H, Osakabe N, Sanbongi C, Yanagisawa R, Inoue K, Yasuda A, Natsume M, Baba S, Ichiishi E, Yoshikawa T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp Biol Med (Maywood). 2004; 229:247–254.

13. Lamaison JL, Petitjean-Freytet C, Carnat A. Rosmarinic acid, total hydroxycinnamic derivatives and antioxidant activity of Apiaceae, Borraginaceae and Lamiceae medicinals. Ann Pharm Fr. 1990; 48:103–108.
14. Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996; 706:181–193.

15. Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986; 16:355–357.
16. Montgomery KC. A test of two explanations of spontaneous alternation. J Comp Physiol Psychol. 1952; 45:287–293.

17. Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat Protoc. 2006; 1:1306–1311.

18. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11:47–60.

19. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.

20. Schmidt HH, Warner TD, Nakane M, Förstermann U, Murad F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol. 1992; 41:615–624.
21. Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of β-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991; 563:311–314.

22. Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990; 250:279–282.

23. Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid β-peptide. Trends Mol Med. 2001; 7:548–554.

24. Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006; 545:51–64.

25. Albarracin SL, Stab B, Casas Z, Sutachan JJ, Samudio I, Gonzalez J, Gonzalo L, Capani F, Morales L, Barreto GE. Effects of natural antioxidants in neurodegenerative disease. Nutr Neurosci. 2012; 15:1–9.

26. Takahashi Y. Handbook of Modern Chinese Medicine II. Tokyo: Yakkyoku Shimbun;1969.
27. Osakabe N, Yasuda A, Natsume M, Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004; 25:549–557.

28. Okuda T, Hatano T, Agata I, Nishibe S. The components of tannic activities in Labiatae plants. I. Rosmarinic acid from Labiatae plants in Japan. Yakugaku Zasshi. 1986; 106:1108–1111.

29. Ishikura N. Anthocyanins and flavones in leaves and seeds of Perilla plant. Agric Biol Chem. 1981; 45:1855–1860.

30. Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer's beta-amyloid fibrils in vitro. J Neurosci Res. 2004; 75:742–750.

31. Makino T, Ono T, Matsuyama K, Nogaki F, Miyawaki S, Honda G, Muso E. Suppressive effects of Perilla frutescens on IgA nephropathy in HIGA mice. Nephrol Dial Transplant. 2003; 18:484–490.

32. Alkam T, Nitta A, Mizoguchi H, Itoh A, Nabeshima T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Aβ(25-35). Behav Brain Res. 2007; 180:139–145.

33. Pereira P, Tysca D, Oliveira P, da Silva Brum LF, Picada JN, Ardenghi P. Neurobehavioral and genotoxic aspects of rosmarinic acid. Pharmacol Res. 2005; 52:199–203.

34. Murai T, Okuda S, Tanaka T, Ohta H. Characteristics of object location memory in mice: behavioral and pharmacological studies. Physiol Behav. 2007; 90:116–124.

35. Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci U S A. 1999; 96:3228–3233.

36. Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006; 443:768–773.

37. Baluchnejadmojarad T, Roghani M, Homayounfar H. Inhibitory effect of high dose of the flavonoid quercetin on amygdala electrical kindling in rats. Basic Clin Neurosci. 2010; 1:57–61.
38. Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002; 32:1050–1060.

39. Cecchi C, Fiorillo C, Baglioni S, Pensalfini A, Bagnoli S, Nacmias B, Sorbi S, Nosi D, Relini A, Liguri G. Increased susceptibility to amyloid toxicity in familial Alzheimer's fibroblasts. Neurobiol Aging. 2007; 28:863–876.

40. Palmer AM, Burns MA. Selective increase in lipid peroxidation in the inferior temporal cortex in Alzheimer's disease. Brain Res. 1994; 645:338–342.

41. Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer's disease. Biomark Med. 2010; 4:27–36.

42. Mattson MP, Begley JG, Mark RJ, Furukawa K. Aβ25-35 induces rapid lysis of red blood cells: contrast with Aβ1-42 and examination of underlying mechanisms. Brain Res. 1997; 771:147–153.

43. Smith MA, Sayre LM, Monnier VM, Perry G. Radical ageing in Alzheimer's disease. Trends Neurosci. 1995; 18:172–176.

44. Choi JY, Cho EJ, Lee HS, Lee JM, Yoon YH, Lee S. Tartary buckwheat improves cognition and memory function in an in vivo amyloid-β-induced Alzheimer model. Food Chem Toxicol. 2013; 53:105–111.

45. Choi JY, Lee JM, Lee DG, Cho S, Yoon YH, Cho EJ, Lee S. The n-butanol fraction and rutin from tartary buckwheat improve cognition and memory in an in vivo model of amyloid-β-induced Alzheimer's disease. J Med Food. 2015; 18:631–641.

46. Lee AY, Yamabe N, Kang KS, Kim HY, Lee S, Cho EJ. Cognition and memory function of Taraxacum coreanum in an in vivo amyloid-β-induced mouse model of Alzheimer's disease. Arch Biol Sci. 2014; 66:1357–1366.

47. Schirrmacher G, Skurk T, Hauner H, Grassmann J. Effect of Spinacia oleraceae L. and Perilla frutescens L. on antioxidants and lipid peroxidation in an intervention study in healthy individuals. Plant Foods Hum Nutr. 2010; 65:71–76.

48. Iuvone T, De Filippis D, Esposito G, D'Amico A, Izzo AA. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-β peptide-induced neurotoxicity. J Pharmacol Exp Ther. 2006; 317:1143–1149.

49. Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996; 271:C1424–C1437.

50. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010; 37:13–25.

51. Jancsó G, Domoki F, Sántha P, Varga J, Fischer J, Orosz K, Penke B, Becskei A, Dux M, Tóth L. β-Amyloid (1-42) peptide impairs blood-brain barrier function after intracarotid infusion in rats. Neurosci Lett. 1998; 253:139–141.

52. Schwab C, McGeer PL. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J Alzheimers Dis. 2008; 13:359–369.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download