Abstract
BACKGROUND/OBJECTIVES
The present study was conducted to examine the inhibitory effect of loquat leaves on MDA-MB-231 cell proliferation and invasion.
MATERIALS/METHODS
Female athymic nude mice were given a subcutaneous (s.c.) inoculation of MDA-MB-231 cells and randomly grouped to receive a s.c. injection of either 500 mg/kg ethanol, water extract or vehicle five times a week. Tumor growth, mitotic rate and necrosis were examined. MDA-MB-231 cells were cultured with DMSO or with various concentrations of loquat water or ethanol extract. Proliferation, adhesion, migration, invasion and matrix metalloproteinase (MMP) activity were examined.
RESULTS
Tumor growth of xenograft nude mouse was significantly reduced by loquat extracts. The results of mitotic examination revealed that loquat extracts reduced tumor cell division. Both ethanol and water extracts significantly inhibited MDA-MB-231 cell proliferation. The protein expression of ErbB3 was significantly down-regulated by loquat leaf extracts. Loquat leaf extracts increased apoptosis of MDA-MB-231 cells following 24 hour incubation and the ethanol extract was more potent in inducing apoptosis than the water extract. Furthermore, loquat extracts inhibited adhesion, migration and invasion of MDA-MB-231 cells. MMP activity was significantly inhibited by loquat extracts.
Breast cancer is the most common disease of malignancy affecting women around the world. For the invasion and metastasis of a primary tumor, neoplastic cells must grow progressively to expand the tumor mass. After expanding the tumor mass, tumor cells undergo a series of events including the disruption of cellular adhesion, adhesion to the extracellular matrix (ECM), migration and invasion of tumor cells into the blood or lymphatic vessels, extravasations out of the vessels, and interactions with new target tissue [12345]. Therefore, inhibition of proliferation, adhesion, migration and invasion is likely to contribute to the prevention of cancer progression.
Loquat (Eriobotrya japonica Lindley), a medicinal plant widely used in Japan and China, is of particular interest due to its anti-cancer properties. The anti-tumorigenic effect of the triterpene acid constituents of loquat leaves has been investigated [6789]. Triterpene acid constituents of loquat leaves have been evaluated for their inhibitory effects on skin tumor promotion in two stage mouse skin carcinogenesis. Eighteen polyphenolic compounds have been isolated and characterized from the loquat leaves and the procyanidin oligomer among the isolated polyphenols was reported to exhibit potent selective cytotoxicity to tumor cell lines [10].
In the present study, we chose the leaf extract due to the fact that it had shown a more potent inhibitory effect on breast cancer cell metastasis in a previous study [11] and further investigated the effect of ethanol and water extracts on in vivo tumor growth and the tumor cell invasion.
The extract of loquat leaves was obtained by macerating freeze-dried leaf powder with 50% ethanol or water for 2 days at room temperature. The respective extract was filtered under reduced pressure and freeze-dried. The yields obtained were 15.6 % for ethanol extract and 19.4 % for water extract.
All reagents were of HPLC grade (Burdick & Jackson, USA). Ursolic acid was purchased from Sigma (St. Louis, USA). A Shimadzu liquid chromatography apparatus consisting of a LC-10AD VP quaternary pump, a CBM-20A VP control system coupled with a RID-10A VP refractive in dex detector, and a SIL-20A injector was used for the HPLC analysis. The temperature of the column was controlled with a CT0-10AS oven (Shimadzu, Tokyo, Japan). Separation was achieved on a 25 cm × 4.6 mm i.d., 5 µm Capcell Pak C18 analytical column provided by Shiseido (Tokyo, JAPAN). Approximately 1 g Eriobotrya japonica leaf extract was dissolved in the mobile phase, exposed to ultrasonic waves for 60 min, and then mobile phase was added to make up a final volume of 100 ml. The resulting solution was used as the test solution. 10 mg of ursolic reference standard was dissolve in methanol to make exactly 10 ml. 1.0 ml of this solution was added to ethanol to make exactly 10 ml, and this solution was used as the ursolic acid standard solution. Table 1 shows the condition of the HPLC analysis.
Four-week-old female athymic nude mice (Balb/c) were purchased from Harlan Laboratories (Indianapolis, IN, USA). The mice were housed in a climate controlled room (22 ± 2℃, 50 ± 10% relative humidity) with a 12 hour light/dark cycle and provided with diet and water ad libitum. The mice were acclimatized to an AIN-93G control diet for a week and then given a subcutaneous (s.c.) inoculation in the bilateral flank of Matrigel containing 2 × 106 MDA-MB-231 cells. Palpable and measurable tumors were found beginning 7-8 days after cells were inoculated and by 3 weeks the tumors had grown at 90% of the injection sites. Three weeks after inoculation, the mice were randomly grouped to receive s.c. injection (N = 10 in each groups) of either 500mg/kg ethanol (ME), water extract (MH) or vehicle (MC) for 4 weeks, five times a week. Food intake, body weight and palpable tumor diameters were measured weekly. Tumor volumes were calculated as (π/6) x [length (mm) x width2 (mm)2]. Seven weeks after cell inoculation, the mice were sacrificed by cervical dislocation and the tumors were dissected for further examination. All experiments were approved by the guidelines of Laboratory Animal Care and Use Committee of Mokpo National University (MNU-IACUC-2014-005).
Isolated tumor mass was divided into small pieces, and fixed in 10% neutral buffered formalin. Then tissues were processed and embedded in paraffin wax, cut into 4 um. The sectioned slides deparaffinized in toluene and rehydrated in alcohol series. Further step, the slides were stained with hematoxylin and eosin (H & E) for microscopic findings. For the microscopic evaluation, the slides were counted on mitotic figures using Leica Application suite software (LAS version 2.8.1, Leica Microsystem) in the high power fields as a 400x fields and analyzed for necrotic portion as a 100x through the 200x fields [1213]. The evaluation of experimental groups are shown as mean ± SD.
The MDA-MB-231 human breast cancer cell line was obtained from the American Type Culture Collection (Rockville, MD). Cells were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco, Gaithersburg), supplemented with penicillin 100units/ml, streptomycin 100 µg/ml and 10% fetal bovine serum, and incubated in a humidified incubator at 37℃ and 5% CO2.
The MDA-MB-231 cells were seeded at a density of 1 × 103/well in 96 well plates. The cells were then treated with ME or MH at various concentrations for 24 hours. After completion of the treatment, the cells were incubated with an MTT solution (MTT:2 mg/ml, Sigma-Aldrich St. Louis MO, USA) for 4 hours at 37℃. The supernatants were aspirated, DMSO (Sigma-Aldrich St. Louis MO, USA) was added to each well. After incubation for 30 min, absorbance was measured at 540 nm using an ELASA.
The MDA-MB-231cells (6 × 104/well) were seeded in 6-well culture plates. Complete medium with DMSO or 75, 100, 150, 200 or 250 µg/ml extract was replaced every other day. Cells cultured in complete medium were harvested on day 5 after treatment and counted.
MDA-MB-231 cells were washed once with cold PBS and scraped into lysis buffer (10 mM Tris-HCl (pH 7.4), 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton-X 100, 10% glycerol, 0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholate, 1 mM PMSF, 5% protease inhibitor cocktail) for 10 min. The lysis mixture was clarified at 13,000 rpm for 20 min at 4℃. The supernatant was collected as the lysate, and the protein concentration was determined by a Bradford protein assay (Sigma, St. Louis MO, USA). The 20 µg of lysate protein was mixed with 5x SDS-PAGE sample buffer containing 60 mM Tris-HCl (pH 6.8), 25% glycerol, 2% SDS, 10% bromophenol, and 5% 2-mercaptoethanol, and heated at 95℃ for 10 min, and separated by SDS-PAGE. After electrophoresis, the proteins were transferred onto a nitrocellulose membrane using a transblot chamber with Tris transfer buffer (0.025 M Tris-HCl, 0.192 M glycine, and 20% MeOH). The membrane was blocked for 1 hour at 4℃ with 5% nonfat milk in TBS-T buffer containing 10 mM Tris-HCl (pH 6.8), 100 mM NaCl, and 0.1% Tween 20. The membranes were then incubated overnight with either of the following primary antibodies from Cell Signaling Technology (Beverly MA, USA): ErbB2 and ErbB3 (all used at 1:1,000 dilutions). In addition, antibody from β-actin was from Santa Cruz Biotechnology (CA, USA) (also used 1:1,000). After washing with TBS-T buffer, the membranes were incubated with horseradish peroxidase conjugated secondary antibody (goat anti-rabbit or mouse anti-goat at 1:2,000) for 1 hour at room temperature. Bands were detected by chemiluminescent substrate (IMGENEX, San Diego CA, USA), and quantified using UVP imaging system (UVP, Upland CA, USA) and Vision Works TMLS analysis software (UVP).
Muse™ Annexin V & Dead Cell Kit was used for the assessments of apoptosis. MDA-MB-231 cells were incubated with PBS, ME or MH for 24 hours. Thereafter, treatment medium was removed and washed with PBS, and collected using trypsin-EDTA. The harvested cells were centrifuged at 1,400rpm for 5 min and re-suspended with complete medium. Muse™ Annexin V & Dead Cell reagent (100 µl) was added to each to re-suspended cell and incubated for 20 min. At the end of 90 min, the re-suspended cell was assayed by Muse Cell Analyzer.
Plates (12 well) were coated with 30 µg Matrigel (Becton Dickinson, Bedford, USA). After rehydration of the Matrigel coated culture plates, MDA-MB-231 cells (2 × 105/ well) in serum free DMEM containing 0.1% BSA were seeded in the presence of DMSO or 75, 100, 150, 200 or 250 µg/ml of extract and incubated for 90 min at 37℃ and 5% CO2. After 90 min, the attached cells were harvested with trypsin-EDTA and then counted with hemocytometer. Each assay was performed in triplicate and repeated in three independent experiments. Values were expressed as the average of the triplicate experiments.
To determine the activity of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) in the conditioned media, MDA-MB-231 cells (2 × 105/ well) were seeded in 6-well plates. After treatment with DMSO or 75, 100, 150, 200 or 250 µg /ml of extract for 24 hours, the supernatants were collected and subjected to gel electrophoresis on 10% running gels containing 0.1% gelatin. The gels were washed in 2.5% Triton X-100 for 30 min, followed by incubation for 20 hours at 37℃ in the incubation buffer containing 50 mM Tris-HCl (pH 7.5), 0.2 M NaCl, 1 mM CaCl2 and 0.2% NaN3. The gels were stained in 0.5% Coomassie brilliant blue (Sigma, St. Louis, USA) then destained with destaining solution (45% methanol and 10% acetic acid) and photographed.
Cells (3 × 105cells ) were seeded into 6 well cell culture plates and cultured in DMEM containing 10% FBS to near confluence. The confluent monolayer was carefully wounded and cellular debris was washed gently with PBS. The wounded monolayer was incubated in DMEM containing 10% FBS, 20 µg/ml fibronectin and DMSO or 75, 100, 150, 200 or 250 µg/ml extract for 24 hours. Migrating cells were examined under 10 × by phase contrast microscope (Olympus, Tokyo, Japan).
Boyden chambers were assembled using 8 µm Falcon transwell inserts (Becton Dikinson, Bedford, MA) as the upper chamber and 12 well plates as the lower chamber. The inserts were coated with Matrigel and re-hydrated with serum free DMEM for 90 min at 37℃ and 5% CO2. Following rehydration, inserts were washed with serum free DMEM. The cells were resuspended in DMEM containing 0.1% BSA and seeded at a concentration of 2 × 105 cells. DMSO or 75, 100, 150, 200 or 250 µg/ml extract was carefully added to the upper chamber. 20 µg of fibronectin was added to the lower chamber. The Boyden chamber was incubated for 24 hours at 37℃ and 5% CO2. After incubation, the cells that transversed the Matrigel and attached to the lower surface of the insert were fixed with 10% formalin and stained with 0.5% crystal violet (Biosesang, Sungnam, Korea). Inserts were examined under phase contrast field microscopy and photographed. Values for invasion are expressed as the number of migrated cells per microscopic field. Each assay was performed in triplicate and repeated in three independent experiments. Values were expressed as the average of the triplicate experiments.
Statistical analysis was carried out using the SPSS program (Version SPSS 21, Chicago, IL, USA). Results are expressed as the mean ± SE for the animal study and the mean ± SD of three independent experiments for the cell based assays. Comparisons are based on one-way ANOVA followed by Duncan's multiple range test. A P-value < 0.05 was considered statistically significant.
The ursolic acid standard was detectable under a wavelength of 370 nm. The ethanol extract of loquat leaves contained more ursolic acid compared to the water extract (Fig. 1).
One week following inoculation of MDA-MB-231 cells, the palpable tumors were observed in the animals for the first time (Fig. 2C). Tumor volumes increased sharply in the control group (MC) four weeks after inoculation. In the loquat ethanol extract (ME) and water extract (MH) treated groups, tumor volumes were not as large as in the control group. No significant difference was observed between the groups treated with ethanol or water extracts. The mean number of mitotic figures was highest in the control group, whereas it was the lowest in the ethanol extract treated group (Fig. 3A). Necrosis of the tumor had occurred in a similar pattern, the necrotic portion at the center, and live cells in the margin of the tumor (Fig. 3B). Tumors in the animals treated with ethanol or water extract showed an increase in the necrotic area.
No cytotoxicity was observed at any concentration following incubation for 24 hours with loquat extracts (Fig. 4A). Loquat leaf extracts inhibited the proliferation of MDA-MB-231 cells at all concentrations following 5 day treatment and ethanol extract showed more potent inhibitory effect than the water extract at concentration above 200 µg/ml (Fig. 4B). Loquat leaf extracts down-regulated the protein expression of ErbBs. Although inhibitory effect of loquat on the expression of ErbB2 was not statistically different, the protein expression of ErbB3 was significantly down-regulated by loquat leaf extracts. The ethanol extract more effectively inhibited the protein expression of ErbB3 at 250 µg/ml (Fig. 5A,B). Loquat leaf extracts increased apoptosis of MDA-MB-231 cells following 24 hour incubation and the ethanol extract was more potent in inducing apoptosis than the water extract (Fig. 5C).
Loquat leaf extract significantly down-regulated the binding of tumor cells to the Matrigel in a dose dependent manner (Fig. 6). The ethanol extract showed a more potent inhibition of adhesion than the water extract at higher concentrations of treatment.
The extracts of loquat leaves had an inhibitory effect on the activities of MMP-9 and MMP-2 (Fig. 7A). Following incubation for 24 hours without the loquat extract, MDA-MB-231 cells migrated and covered the wound area. In the presence of loquat leaf extracts, MDA-MB-231 cell migration was significantly inhibited (Fig. 7B).
Loquat leaf extracts dose dependently suppressed the invasion of MDA-MB-231 cells (Fig. 8). The ethanol extract more potently inhibited the invasion of MDA-MB-231 breast cancer cells than the water extract.
In order to expand a tumor mass for invasion and metastasis, tumor cells must grow progressively. Hence, inhibition of tumor growth is the first step in the prevention of invasion and metastasis [3]. In the present study, loquat leaf extract inhibited tumor growth in MDA-MB-231 xenograft nude mice, and the ethanol extract reduced the number of mitotic figures more potently compared to the water extract. Mitotic figure can explain both mitotic arrest and anti-proliferation effects [14]. Consequently, the reduction in tumor volume and mitotic figures following loquat extract treatment implied its cancer preventive effect. The extract of loquat leaves also showed an inhibitory effect on the proliferation of MDA-MB-231 cells. To verify the involvement of ErbBs in MDA-MB-231 cell proliferation, we determined the protein expression of human epidermal growth factor receptors (ErbBs, commonly referred to as HERs). ErbBs belong to the superfamily of receptor tyrosine kinase (RTKs) and overexpression of ErbBs has been found in cancer [15]. In the present study, loquat leaf extract significantly decreased the protein expression of ErbB 3. It has been shown that ErbB3 is required for proliferation of ErbB2-driven breast cancer cells [16]. Furthermore, ErbB3/ ErbB2 dimerization activates Akt signaling pathway, which is crucial for cell survival and is dysregulated in most cancers [1617]. Therefore, down regulation of ErbB3 protein expression following loquat leaf extract treatment may be one of mechanisms for the inhibition of MDA-MB-231 cell proliferation. The inhibitory effect of the ethanol extract was more potent than that of the water extract.
Triterpenoids have been isolated from loquat leaves, and ursolic acid has been identified as an active component [9101819]. Ursolic acid is also used as a standard for verification test of loquat leaves in thin layer chromatography in the Korean pharmacopoeia [20]. Moreover, usolic acidic showed a delay in two-stage skin carcinogenesis model in mice [19]. Based on these reports, we determined the content of ursolic acid in the extract of loquat leaves used in the present study. HPLC analysis results showed that ursolic acid was more abundant in the ethanol extract compared to the water extract. Previous reports have revealed that ursolic acid inhibited proliferation of tumor cells via the induction of apoptosis [212223]. In our study, loquat extract increased the apoptosis of MDA-MB-231 cells and the ethanol extract induced apoptosis more potently compared to the water extract. However, we also observed that the water extract of loquat leaves, in which ursolic acid was hardly detected, inhibited cell proliferation. Therefore, besides ursolic acid, loquat leaves may contain other compounds that contribute to the inhibition of tumor cell proliferation. Loquat leaves are known to contain polyphenols, which have selective cytotoxicity against cancer cell lines relative to normal cells [10].
We observed that tumors in the animals treated with the ethanol or the water extract of loquat had an increased necrotic area (Fig. 3B). Ursolic acid has been previously reported to suppress angiogenesis [2223]. Tumor necrosis is caused by chronic ischemia within tumors, blood shunting to other sites and/or rapid tumor cell growth overtaking the rate of neovascularization in a given area [13]. Hence, in our study, suppression of angiogenesis by ursolic acid may be one of mechanisms by which the ethanol extract induced necrosis of the tumors.
The adhesion of cancer cells to the extracellular matrix (ECM) is required to initiate metastatic invasion [24]. We observed the inhibitory effect of loquat leaf extract on the MDA-MB-231 cell attachment to the ECM using a Matrigel binding assay. Following adhesion of cancer cells to the ECM, local invasion of the host stroma occurs as a result of the up-regulated expression of molecules that allow the cancer cells to digest the ECM around them and migrate [24]. To verify the involvement of MMPs in local invasion and migration, we determined the activity of MMP-2 and MMP-9. Both the ethanol and water extracts effectively inhibited MMP-2 and MMP-9 activity. MMP-2 and MMP-9 have capacities for tissue remodeling via ECM basement membrane degradation and angiogenesis induction [25]. These capacities of MMPs are related to tumor invasion and metastasis. Taken together, the results presented here support the implication of MMPs in cell migration and invasion. Ursolic acid has been shown to suppress migration and invasion by modulating the expression of c-Jun N-terminal kinase and Akt [26]. Our present results show that the ethanol extract contained more ursolic acid, and more potently suppressed the invasion and migration of breast cancer cells than the water extract. The Boyden chamber model was used to observe the overall process of cell invasion. To successfully penetrate the Boyden chamber, cells must successfully adhere, degrade and transverse the Matrigel coated insert [27]. In our Boyden chamber model, loquat leaf extracts suppressed the invasion of MDA-MB-231 cells (Fig. 8).
In conclusion, here we demonstrated that loquat leaf extract also suppressed tumor cell proliferation in the MDA-MB-231 xenograft animal model and reduced the invasiveness of MDA-MB-231 human breast cancer cells. The inhibitory effects on the adhesion, migration and MMP activities are possible mechanisms for the inhibition of invasiveness. The ethanol extract was more effective than the water extract on the inhibition of proliferation, adhesion, invasion, and MMP activities in the MDA-MB-231 cells. Our present results provide a theoretical foundation for the possibility of loquat leaf extract as a potential agent in the treatment of cancer metastasis.
Figures and Tables
 | Fig. 1HPLC chromatograms of the ursolic standard (A) and the Eriobotrya japonica water (B) and ethanol (C) extracts. |
 | Fig. 2Effect of Eriobotrya japonica on the body weight (A), food intake (B) and tumor volume (C) of athymic nude mice.Three weeks after inoculation, nude mice were randomly grouped to receive s.c. injection of either 500mg/kg ethanol (ME), water extract (MH) or vehicle (MC) for 4 weeks, five times a week. The weight, food intake and tumor volume were measured weekly. MC: PBS s.c., ME: 50% ethanol extract (500 mg/kg) s.c., MH: water extract (500 mg/kg) s.c.. Means with the same letter are not significantly different based on "one-way ANOVA followed" by Duncan's multiple range test (P < 0.05)
|
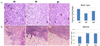 | Fig. 3Effect of Eriobotrya japonica on the mitotic figures (A) and the necrosis (B) of tumors in MDA-MB231 xenograft nude mice.Formalin-fixed, paraffin-embedded tumor tissues were sectioned at 4µmand stained with H&E for microscopic findings. MC: PBS s.c., ME: 50% ethanol extract (500 mg/kg) s.c., MH: water extract (500 mg/kg) s.c. arrow: mitotic figure
|
 | Fig. 4Effect of Eriobotrya japonica on the cell availability (A) and proliferation (B) of MDA-MB-231 cells.(A) Cells were seeded at a density of 1 × 103cells/well in 96 well plates and treated with different concentration of ME or MH for 24h. After completion of the treatment, the cell viability was determined by MTT assay. (B) Cells were seeded in 6 well plates at a concentration of 6 × 104cells/well. Complete medium with PBS, ME or MH was replaced every other day. Cells were harvested on day 5 after treatment and were counted. Each assay was performed in triplicate and repeated in three independent experiments. ME: 50% ethanol extract, MH: water extract Means with the same letter are not significantly different based on "one-way ANOVA followed" by Duncan's multiple range test (P < 0.05)
|
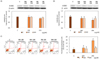 | Fig. 5Effect of Eriobotrya japonica on the apoptosis of MDA-MB-231 cells.(A), (B) Cells were treated with PBS, ME or MH for 24h. Cells were lysed and determined protein expression of ErbB2(A) and ErbB(B) by western blotting analysis. (C) MDA-MB-231 cells were incubated for 24h and cell apoptosis was evaluated by Muse Cell Analyzer. ME: 50% ethanol extract, MH: water extract Means with the same letter are not significantly different based on "one-way ANOVA followed" by Duncan's multiple range test (P < 0.05)
|
 | Fig. 6Effect of Eriobotrya japonica on the adhesion of MDA-MB-231 cells.Cells were added to the matrigel coated plates and incubated for 90min in the presence of PBS. ME or MH and attached cells were harvested and counted. Each assay was performed in triplicate and repeated in three independent experiments. ME: 50% ethanol extract, MH: water extract Means with the same letter are not significantly different based on "one-way ANOVA followed" by Duncan's multiple range test (P < 0.05)
|
 | Fig. 7Effect of Eriobotrya japonica on the MMP activities (A) and the migration (B) of MDA-MB-231 cells.(A) MDA-MB-231 cells (2 × 105/ well) were seeded in 6-well plates. After treatment with DMSO or 75, 100, 150, 200 or 250 µg /ml of extract for 24 hours, the supernatants were collected and subjected to gel electrophoresis. (B) MDA-MB-231 cells were seeded into 6 well plates and cultured to near confluence. Confluent monolayer was carefully wounded and washed cellular debris gently. The wounded monolayer was incubated in 10% FBS DMEM containing 20 µg/ml fibronectin and DMSO, ME or MH for 24h. ME: 50% ethanol extract, MH: water extract
|
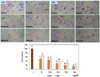 | Fig. 8Effect of Eriobotrya japonica on the invasion of MDA-MB-231 cells.Boyden chamber was used for the invasion assay. MDA-MB-231 cells (2 × 105) were seeded and DMSO , ME or MH was carefully added to the upper chamber. 20 µg of fibronectin was added to the lower chamber. The Boyden chamber was incubated for 24 hours at 37℃ and 5% CO2. Following incubation, the cells that transversed the Matrigel and attached to the lower surface of the insert were fixed and stained. ME: 50% ethanol extract, MH: water extract Means with the same letter are not significantly different based on "one-way ANOVA followed" by Duncan's multiple range test (P < 0.05)
|
References
1. Radisky ES, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010; 15:201–212.

2. Tomaskovic-Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009; 11:213.

3. Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003; 3:453–458.

4. Geho DH, Bandle RW, Clair T, Liotta LA. Physiological mechanisms of tumor-cell invasion and migration. Physiology (Bethesda). 2005; 20:194–200.

6. Kim MS, You MK, Rhuy DY, Jeong KS, Kim YJ, Baek HY, Kim HA. Oral administration of loquat suppresses DMBA-induced breast cancer in rats. Food Sci Biotechnol. 2011; 20:491–497.

7. Liang ZZ, Aquino R, Feo VD, Simone FD, Pizza C. Polyhydroxylated Triterpenes from Eriobotrya japonica. Planta Med. 1990; 56:330–332.

8. Shimizu M, Uemitsu N, Shirota M, Matsumoto K, Tezuka Y. A new triterpene ester from Eriobotrya japonica. Chem Pharm Bull (Tokyo). 1996; 44:2181–2182.

9. Banno N, Akihisa T, Tokuda H, Yasukawa K, Taguchi Y, Akazawa H, Ukiya M, Kimura Y, Suzuki T, Nishino H. Anti-inflammatory and antitumor-promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol Pharm Bull. 2005; 28:1995–1999.

10. Ito H, Kobayashi E, Takamatsu Y, Li SH, Hatano T, Sakagami H, Kusama K, Satoh K, Sugita D, Shimura S, Itoh Y, Yoshida T. Polyphenols from Eriobotrya japonica and their cytotoxicity against human oral tumor cell lines. Chem Pharm Bull (Tokyo). 2000; 48:687–693.

11. Kim MS, You MK, Rhuy DY, Kim YJ, Baek HY, Kim HA. Loquat (Eriobotrya japonica) extracts suppress the adhesion, migration and invasion of human breast cancer cell line. Nutr Res Pract. 2009; 3:259–264.

12. Park JK, Ki MR, Lee EM, Kim AY, You SY, Han SY, Lee EJ, Hong IH, Kwon SH, Kim SJ, Rando TA, Jeong KS. Losartan improves adipose tissue-derived stem cell niche by inhibiting transforming growth factor-β and fibrosis in skeletal muscle injury. Cell Transplant. 2012; 21:2407–2424.

13. Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999; 79:991–995.

14. Zhang ZR, Al Zaharna M, Wong MM, Chiu SK, Cheung HY. Taxifolin enhances andrographolide-induced mitotic arrest and apoptosis in human prostate cancer cells via spindle assembly checkpoint activation. PLoS One. 2013; 8:e54577.

15. Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005; 5:341–354.

16. Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003; 100:8933–8938.

17. Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009; 21:177–184.

19. Taniguchi S, Imayoshi Y, Kobayashi E, Takamatsu Y, Ito H, Hatano T, Sakagami H, Tokuda H, Nishino H, Sugita D, Shimura S, Yoshida T. Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry. 2002; 59:315–323.

20. Ministry of Food and Drug Safety (KR). The Korean Pharmacopoeia. 10th ed. Seoul: Yakup Daily;2013. p. 50–51.
21. Kim KH, Seo HS, Choi HS, Choi I, Shin YC, Ko SG. Induction of apoptotic cell death by ursolic acid through mitochondrial death pathway and extrinsic death receptor pathway in MDA-MB-231 cells. Arch Pharm Res. 2011; 34:1363–1372.

22. Shanmugam MK, Dai X, Kumar AP, Tan BK, Sethi G, Bishayee A. Ursolic acid in cancer prevention and treatment: molecular targets, pharmacokinetics and clinical studies. Biochem Pharmacol. 2013; 85:1579–1587.

23. Shan JZ, Xuan YY, Ruan SQ, Sun M. Proliferation-inhibiting and apoptosis-inducing effects of ursolic acid and oleanolic acid on multi-drug resistance cancer cells in vitro. Chin J Integr Med. 2011; 17:607–611.

24. Meng Q, Goldberg ID, Rosen EM, Fan S. Inhibitory effects of Indole-3-carbinol on invasion and migration in human breast cancer cells. Breast Cancer Res Treat. 2000; 63:147–152.

25. Johansson N, Ahonen M, Kähäri VM. Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci. 2000; 57:5–15.

26. Yeh CT, Wu CH, Yen GC. Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling. Mol Nutr Food Res. 2010; 54:1285–1295.

27. Cos S, Fernández R, Güézmes A, Sánchez-Barceló EJ. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998; 58:4383–4390.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download