Abstract
BACKGROUND/OBJECTIVES
Citrus and its peels have been used in Asian folk medicine due to abundant flavonoids and usage of citrus peels, which are byproducts from juice and/or jam processing, may be a good strategy. Therefore, the aim of this study was to examine antioxidant and anti-inflammatory effects of bioconversion of Jeju Hallabong tangor (Citrus kiyomi × ponkan; CKP) peels with cytolase (CKP-C) in RAW 264.7 cells.
MATERIALS/METHODS
Glycosides of CKP were converted into aglycosides with cytolase treatment. RAW 264.7 cells were pre-treated with 0, 100, or 200 µg/ml of citrus peel extracts for 4 h, followed by stimulation with 1 µg/ml lipopolysaccharide (LPS) for 8 h. Cell viability, DPPH radical scavenging activity, nitric oxide (NO), and prostagladin E2 (PGE2) production were examined. Real time-PCR and western immunoblotting assay were performed for detection of mRNA and/or protein expression of pro-inflammatory mediators and cytokines, respectively.
RESULTS
HPLC analysis showed that treatment of CKP with cytolase resulted in decreased flavanone rutinoside forms (narirutin and hesperidin) and increased flavanone aglycoside forms (naringenin and hesperetin). DPPH scavenging activities were observed in a dose-dependent manner for all of the citrus peel extracts and CKP-C was more potent than intact CKP. All of the citrus peel extracts decreased NO production by inducible nitric oxide synthase (iNOS) activity and PGE2 production by COX-2. Higher dose of CKP and all CKP-C groups significantly decreased mRNA and protein expression of LPS-stimulated iNOS. Only 200 µg/ml of CKP-C markedly decreased mRNA and protein expression of cyclooxygenase-2 in LPS-stimulated RAW 264.7 cells. Both 100 and 200 µg/ml of CKP-C notably inhibited mRNA levels of interleukin-1β (IL-1β) and IL-6, whereas 200 µg/ml CKP-C significantly inhibited mRNA levels of TNF-α.
CONCLUSIONS
This result suggests that bioconversion of citrus peels with cytolase may enrich aglycoside flavanones of citrus peels and provide more potent functional food materials for prevention of chronic diseases attributable to oxidation and inflammation by increasing radical scavenging activity and suppressing pro-inflammatory mediators and cytokines.
Inflammation is a local defensive reaction of a host to cellular injury or infection with biological, chemical, or physical stimuli [12]. During early stage of inflammation, macrophages release a variety of pro-inflammatory cytokines and inflammatory mediators. For example, lipopolyssacharide (LPS)-activated RAW 264.7 cell line stimulates pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor (TNF)-α, monocyte chemoattractant protein-1, and other inflammatory mediators such as nitric oxide (NO) and prostaglandin E2 (PGE2), which are synthesized by iinducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), respectively [34]. As long as they are under control, inflammatory responses have a beneficial role in the eradication of bacteria. Once out of control, deregulated inflammation leads to abundant production of pro-inflammatory cytokines and mediators, which can result in tissue injury and organ damage [12].
Jeju Hallabong tangor (Citrus kiyomi × ponkan), a seedless citrus fruit, is a commonly consumed fruit in Korea. Citrus kiyomi × ponkan, a hybrid variety, was bred by crossing between Kiyomi (Citrus kiyomi) and Ponkan (Citrus reticulate) in Japan, 1972 [56]. Mature Citrus fruit peels have abundant flavonoids among phytochemicals and have been used in Asian medicine for centuries to improve bronchial and asthmatic conditions and to prevent cancer, inflammatory and degenerative diseases [78]. Citrus flavonoids have been shown to improve antioxidant activity [9], glucose tolerance, and inhibit hydroperoxide production [10]. In addition, citrus peels were also reported to inhibit pro-inflammatory effects on hepatitis C virus [11], certain bacteria and fungi [12], and tumor growth in a murine renal cell carcinoma model [13]. Citrus flavonoids have bioactive phytochemicals, which are commonly classified as the six groups; flavones, flavanones, flavonols, isoflavones, anthocyanidins and flavanols according to their molecular structures [14]. Flavanones are the main forms in citrus flavonoids and are much richer in citrus peels and seeds than in citrus tissues. Citrus flavanones occur as glycoside (naringin, narirutin, hesperidin) or aglycoside (naringenin, hesperetin) forms [15]. The aglycoside forms are more effectively absorbed than glycoside forms [16], resulting in higher concentrations of aglycoside forms in plasma, urine, and bile [15].
Citrus peels, which are byproducts of manufacturing processes of juices, jams, jellies, and other fruit preserves, contain many flavanones. The sugar groups are cleaved off by intestinal enzymes prior to glucuronidation, followed by cleavage of the β-glycosidic bond by microflora of the large intestine [15]. After hydrolysis, flavanone rutinoside forms are converted into flavanone aglycoside forms, causing faster absorption and higher concentrations in the body. In addition, flavanone aglycoside forms showed higher biological activity [15]. For example, fermentation of ginseng extracts with β-glucosidase or cytolase exerted substantial anti-inflammatory activity by converting massive amounts of ginsenoside forms in murine macrophages [17] and anti-cancer in human colon adenocarcinoma [18]. Therefore, bioconversion of citrus peel extracts with cytolase, a pectolytic mixture of various glycosidases extracted from Aspergillus niger, is considered to boost more active compounds against pro-inflammation.
Although the anti-inflammatory effect of citrus flavonoids has been gradually established, peel extracts of Citrus kiyomi × ponkan and its flavonoids after bioconversion have not yet been evaluated in inflammatory processes. In this study, we attempted to determine whether Citrus kiyomi × ponkan peel extracts after bioconversion with cytolase enhanced the antioxidant and anti-inflammatory effects by analyzing 1,1-diphenyl-2-picrylhyorazyl (DPPH) radical scavenging activity, pro-inflammatory cytokines and other inflammatory mediators in LPS-stimulated RAW 264.7 cells.
The peel extracts of Citrus kiyomi × ponkan (CKP) and its peel extracts after bioconversion by cytolase PCL5 (CKP-C; DSM food specialties, Heerlen, Netherlands) were supplied by BK Bio Co., Ltd. (SungNam, South Korea). CKP peel was supplied by Institute of BK Bio Co. (Jeju Island, South Korea). Briefly, dried CKP peel (100 g) with 0.5% (w/w) cytolase PCL5 (190 units/g) was extracted in 2,000 ml of d-H2O by incubation at pH 4.0, 60℃ for 14 h and enzyme-treated peels were filtered using filter paper with 6 µm pore size (No. 3; Whatman, Little Chalfont, England). Filtered elutes were extracted in 8,000 ml of 80% ethanol at 60℃ for 8 h and then evaporated to freeze-dried powder. The flavonoid compositions of CKP and CKP-C were analyzed by high-performance liquid chromatography (HPLC; YL9100HPLC, Younglin, Anyang, South Korea) and separated on an Eclipse plus C18 column (4.6 mm×250 mm; Agilent Co., Santa Clara, CA, USA). The UV spectrum was recorded at 280 nm with internal standards such as narirutin, naringin, naringenin, hesperidin, and hesperetin (Sigma-Aldrich Co., St. Louis, MO, USA). Bioconversion rate was calculated with the flavonoid contents of glycone and aglycone forms in the following equation. Bioconversion rate (%) = {aglycone/(aglycone + glycone)} × 100.
RAW 264.7 cell line was purchased from the Korea Cell Line Bank (KCLB, Seoul, South Korea). Cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco) and 1% penicillin-streptomycin (PS; Gibco) in a 5% CO2 atmosphere. In all experiments, confluent cells were treated with 0, 100, or 200 µg/ml of CKP and CKP-C for 4 h, followed by 1 µg/ml of LPS for 8 h.
RAW 264.7 cells were plated at a density of 4 × 104 cells per well in a 96-well plate. The 80% confluent cells were pretreated with 0-500 µg/ml of CKP and CKP-C for 24 h. The medium was replaced with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Amresco, Solon, OH, USA) dye solution [5 mg/ml in phosphate-buffered saline (PBS)] at 37℃ for 4 h. The supernatant was decanted and the formazan salts were dissolved with 500 µl of isopropanol. Absorbance was measured at 570 nm using a spectrophotometer (Gen5.2; Biotek, Winooski, VT, USA).
The antioxidant activity of the extracts was determined on the basis of the scavenging activity of the stable DPPH (Sigma) radical according to the modified method of Brand-Williams et al. [19]. 120 µl of 0.1 mM DPPH solution in methanol was mixed with 80 µl of CKP and CKP-C solution of different concentrations (0, 100, and 200 µg/ml). A series of known concentrations (0-50 µg/ml) of quercetin (Sigma) was used as a reference standard. Absorbance was measured at 515 nm after 30 min in dark using a spectrophotometer (Gen5.2; Biotek). The inhibition % was calculated using the following formula.
Inhibition % of DPPH radical activity = (1-Abs/Abc) × 100 where Abs is the absorbance of the sample and Abc is the absorbance of the control.
NO release was determined by the accumulation of nitrite (NO2-), the stable oxidation product of NO, using the NO-specific colorimetric assay kit (Cayman Chemical, Ann Arbor, MI, USA). Briefly, nitrate reductase was added to 100 µl of media with incubation at room temperature for 30 min for the conversion of nitrate into nitrite. After incubation, 2,3-diaminonaphthotriazole was added as an acidic solution, followed by NaOH which enhances the detection of the fluorescent product, 1(H)-naphthotriazole. Absorbance was measured at 540 nm using a fluorescence spectrophotometer (Victor™ X; PerkinElmer, Waltham, MA, USA). Each sample was normalized to cell protein content and a serial dilution of nitrite standard was prepared for each experiment.
PGE2 levels in the medium were measured using an ELISA kit (R&D Systems, Minneapolis, MN, USA). In brief, 50 µl of cell culture media or standard solution were incubated with the diluted enzyme conjugate of PGE2 at room temperature for 1 h. After incubation, 50 µl of PGE2 conjugate was added, followed by incubation at room temperature for 2 h. After washing three times, 200 µL of substrate solution was added to generate an optimal color after 30 min. Following addition of 100 µl of stop solution containing 0.18 M H2SO4, absorbance was measured at 450 nm using a spectrophotometer (Gen5.2; Biotek). PGE2 concentration was calculated corresponding to the mean absorbance from PGE2 standard curve.
Isolation of total RNA was performed using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA (2 µg) was reverse-transcribed using reverse transcription master premix (ELPIS, Daejeon, South Korea). Real time-PCR (CFX96 Touch; Bio-rad, Hercules, CA, USA) was performed using the following primers (Bioneer, Daejeon, South Korea): mouse iNOS sense 5'-ACGCTTCACTTCCAATGC-3', backward 5'-GGGCTCTGTTGAGGTCTA-3', COX-2 sense 5'-CTCTCTGAACTATGGTGTGAAC-3', anti-sense 5'-GTCTGCTTTATGCGTAAATTCC-3', IL-6 sense 5'-GTCCTTCAGAGAGATACAG AAAC-3', anti-sense 5'-GGTCCTTAGCCACTCCTT-3', IL-1β sense 5'-TTGACGGACCCCAAAAGAT-3', anti-sense 5'-GATGTGCTGCTGCGAGATT-3', TNF-α sense 5'-CTCTTCAAGGGACAAGGC-3', anti-sense 5'-CTCCTGGTATGAGATAGCAAAT-3', β-actin sense 5'-GGGAAGGTGACAGCATTG-3', anti-sense 5'-ATGAAGTATTAAGGCGGAAGATT-3'. The PCR results were normalized against results obtained with β-actin primers.
Whole cell lysates (20 µg protein each) were resolved by 10% SDS-PAGE, transferred to a polyvinylidene difluoride membrane (Roche, Basel, Switzerland), and probed with primary antibodies specific to inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), or β-actin (Santa Cruz Biotechnology, Dallas, TX, USA). After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz). Immunodetection was performed using a chemiluminescence method (SuperSignal; Pierce Biotechnology, Rockford, IL, USA) and then normalized with β-actin.
For all experiments, statistical analysis using IBM SPSS statistics programs (Ver. 20, IBM-SPSS, Armonk, NY, USA) was performed by one-way ANOVA followed by post-hoc analysis using the Tukey test to determine differences between experimental groups. A value of P < 0.05 was considered statistically significant.
The flavonoid contents of HPLC chromatograms showed that CKP and CKP-C have 24.66% and 23.08% of total flavonoids. Cytolase, a pectolytic mixture of various glycosidases, increased aglycoside forms by hydrolysis of rhamnose-glucose (rutinose) from flavanone rutinoside forms. There were substantial bioconversion of narirutin to naringenin and of hesperidin to hesperetin, respectively. Narirutin decreased from 3.20% in CKP to 0.16% in CKP-C and instead, naringenin increased from 0.01% in CKP to 8.82% in CKP-C. In addition, hesperidin decreased from 21.42% in CKP to 1.72% in CKP-C, whereas hesperetin increased from 0.00% in CKP to 12.38% in CKP-C (Table 1). Cytolase and/or bioconversion method appear to be suitable for further studies and industrial applications, since bioconversion rates were 0.04% for CKP and 91.85% for CKP-C, respectively. Naringin, a flavone of neohesperidoside form (rhamnosyl-α-1,2-glucose) of naringenin, was negligible in both CKP and CKP-C, suggesting that naringin is not a major flavonoid form in CKP.
To examine the antioxidant and anti-inflammatory effect of CKP and CKP-C, we first evaluated the cytotoxicity of CKP and CKP-C in RAW 264.7 cells by treatment with various concentrations (0-500 µg/ml) of CKP and CKP-C for 24 h. No inhibitory effects on cell viability were observed in cells treated with up to 500 µg/ml of CKP, whereas CKP-C at higher concentrations of 300-500 µg/ml induced significant cell toxicity (Fig. 1). Therefore, we determined to use 100 and 200 µg/ml of the extracts in further experiments to avoid cell toxicity.
To assess the antioxidant activity of the extracts, we measured DPPH radical scavenging activity of CKP and CKP-C. Citrus peel extracts induced a significant increase in DPPH radical scavenging activity at 100 and 200 µg/ml concentrations compared to the control (Fig. 2). Higher dose of CKP-C showed more potent radical scavenging activity compared to lower dose of the extracts and CKP (Fig. 2).
To determine effects of CKP and CKP-C on macrophage function, we examined NO and PGE2 generation in RAW 264.7 cells. Treatment with 1 µg/ml of LPS resulted in significantly increased production of NO and PGE2 by 3-5 fold, which was significantly inhibited by all citrus groups (Fig. 3). Treatment with 100 and 200 µg/ml of CKP and CKP-C resulted in significantly decreased production of NO and PGE2 compared to those of LPS-stimulated cells. NO level of CKP-C and PGE2 levels of both CKP and CKP-C at a dose of 200 µg/ml were significantly similar to those of control without LPS treatment (Fig. 3). This result indicated that citrus peel extracts decreased NO and PGE2 over-production in macrophages.
To determine whether CKP and CKP-C regulate iNOS and COX-2 to decrease inflammatory mediators, we examined mRNA and protein expression of iNOS and COX-2. Treatment with 1 µg/ml of LPS resulted in markedly increased mRNA levels as well as protein expression of iNOS and COX-2. In terms of mRNA levels, 200 µg/ml of CKP and all CKP-C groups significantly decreased mRNA levels of iNOS but only 200 µg/ml of CKP-C markedly inhibited mRNA levels of COX-2, compared to those of LPS-stimulated genes (Fig. 4). Regarding protein expression, treatment with both CKP and CKP-C resulted in markedly decreased LPS-induced expression of iNOS and COX-2 (Fig. 5).
To determine whether CKP and CKP-C inhibited pro-inflammatory cytokines, mRNA levels of IL-1β, IL-6, and TNF-α were measured. There were no changes in mRNA levels of IL-1β, IL-6, and TNF-α in CKP group but a decrease in TNF-α mRNA levels at 200 µg/ml in the CKP group, compared to the LPS-stimulated group (Fig. 6). However, bioconversion of CKP with cytolase completely inhibited mRNA levels of IL-1β and IL-6 at 100 and 200 µg/ml doses and TNF-α mRNA levels at a dose of 200 µg/ml (Fig. 6).
In this study, we compared the antioxidant and antiinflammatory effect of Citrus kiyomi × ponkan peel extracts before and after bioconversion with cytolase. Bioconversion of citrus peels with cytolase resulted in decreased narirutin and hesperidin, indicating that cytolase, a mixture of various glycosidases, deglycosylated certain citrus flavonoid glycosides, particularly flavanone rutinoside forms and instead, increased naringenin and hesperetin.
Treatment of RAW 264.7 macrophages with citrus peel extracts resulted in markedly increased DPPH radical scavenging activity and cytolase-treated CKP had far more scavenging activity than CKP alone, suggesting that treatment with citrus peel extracts and cytolase enhances antioxidant activity of CKP. Although all of the citrus extracts decreased production of NO and PGE2 and molecular expression of iNOS and COX-2 in LPS-stimulated RAW 264.7 cells, bioconversion of CKP peel extracts with cytolase markedly suppressed mRNA levels and protein expression of iNOS and COX-2, consequently resulting in less NO and PGE2 production. In addition, cytolase-treated CKP completely inhibited mRNA levels of IL-1β and IL-6 at 100 and 200 µg/ml concentrations and TNF-α mRNA levels at a 200 µg/ml concentration. These results suggest that bioconversion of CKP peel extracts with cytolase, which causes formation of aglycoside flavonoids, enhances their anti-inflammatory effects by suppression of NO and PGE2 production and pro-inflammatory mediators and cytokines by regulating their relevant genes at molecular levels in macrophages.
HPLC analysis showed that treatment of CKP peel with cytolase catalyzed hydrolysis of flavanone rutinoside forms (narirutin and hesperidin) into aglycoside forms (naringenin and hesperetin) and rutinose (rhamnosyl-α-1,6-glucose). This effect is similar to that of β-glucosidases in the gastrointestinal tract. Beta-glucosidases catalyze the hydrolysis of β-glucosidic bonds, particularly terminal non-reducing residues in D-glucoside with release of glucose. Their action causes deglycosylation of various flavonoid glycosides, particularly flavanone rutinoside in citrus products. The glycoside forms have a more stable structure and are less degraded by enzymes and/or colonic microbiota [20]. Therefore, the aglycoside forms have greater bioavailability than glycoside forms [16], resulting in higher concentrations of aglycoside forms in plasma, urine, and bile [15]. In addition to bioavailability, the removal of rutinose improves the bioactivity of glycoside forms [1520]. For example, after elimination of sugar tailoring, aglycoside flavonoids exert more potential activities against anti-virus, anti-bacteria, and anti-parasite [21]. Hesperetin was also reported to inhibit Helicobacter pylori activity [22] and intracellular replication of human virus [23].
Antioxidants play an important role in prevention of reactive free radicals and therefore human diseases. The DPPH assay shows the antioxidant activity of water soluble phenolics because of their hydrogen-donating ability [2024]. Citrus peel extracts induced significantly increased DPPH radical scavenging activity and higher dose of CKP-C showed more potent radical scavenging activity compared to lower dose of the extracts (Fig. 2). Because the DPPH radical scavenging ability of 200 µg/ml CKP-C was significantly similar to that of quercetin, it was evident that these extracts functioned as free radical scavengers and as potential antioxidants to prevent radical-related damage including inflammation and that CKP-C was more potent than intact CKP. This result might be, in part, due to substantial bioconversion of narirutin to naringenin and of hesperidin to hesperetin, respectively. It has been reported that aglycoside forms such as naringenin and hesperetin have more potent antioxidant activity than glycoside forms such as narirutin, naringin, and hesperidin [578]. For example, bioconversion of Citrus unshiu peel extracts with cytolase increased DPPH radical scavenging ability in a dose-dependent manner compared to intact Citrus unshiu peel extracts in LPS-stimulated RAW 264.7 cells [5]. This study showed that CKP-C triggered more DPPH radical scavenging activity, suggesting that bioconversion of citrus peel with cytolase induced a greater antioxidant effect due to higher amounts of aglycoside forms and their reducing power [2024].
During the inflammatory response, iNOS generates a considerable amount of NO, a well-known pro-inflammatory mediator. NO inhibition has been studied as an effective therapeutic strategy for inflammation-related diseases [12]. In this study, we showed that all citrus peel extracts decreased NO and PGE2 generation as a result of decreased mRNA levels and protein expression of iNOS and COX-2 in LPS-activated macrophages. In addition, CKP-C efficaciously suppressed mRNA and protein expression of LPS-induced COX-2 compared to those of CKP. It was reported that iNOS levels were inhibited by both naringenin and hesperetin and COX-2 levels were inhibited by naringenin, not by hesperetin in LPS-stimulated macrophages [1825]. Because bioconversion with cytolase increased amounts of naringenin and hesperetin, the CKP-C group had more inhibitory effects on iNOS rather than on COX-2. In addition, no inhibitory effect of COX-2 by hesperetin [1825] appeared to trigger more iNOS inhibition than COX-2 inhibition. Therefore, these results suggest that bioconversion of CKP with cytolase may contribute to a more potent anti-inflammatory agent by more suppression of NO by iNOS rather than PGE2 by COX-2, which are associated with anti-inflammatory regulation in LPS-stimulated RAW 264.7 cells.
Activated macrophages and lymphocytes secrete pro-inflammatory cytokines and control the inflammatory responses to inflammatory diseases [1226]. Because iNOS and COX-2 are induced by pro-inflammatory cytokines, we examined mRNA levels of IL-1β, IL-6, and TNF-α in LPS-stimulated RAW 264.7 cells. Kang reported that citrus peel extract triggered more inhibition of IL-6 levels and less inhibition of TNF-α and IL-6 in LPS-induced RAW 264.7 cells [27]. Bioconversion of CKP with cytolase resulted in complete inhibition of mRNA levels of IL-1β and IL-6. Bioconversion of CKP with cytolase and higher dose of citrus peel extracts resulted in a greater decrease in TNF-α mRNA levels. Similarly, it was reported that Citrus aurantium L. extracts inhibited mRNA and protein expression of IL-6 and TNF-α as well as iNOS and COX-2 in a dose-dependent manner [28] and that Citrus reticulate extract decreased NO production via iNOS expression at mRNA and protein levels [29] in LPS-stimulated RAW 264.7 cells. Regarding bioconversion, bioconversion of Citrus unshiu peel extracts with cytolase was more potent than intact Citrus unshiu peel in NO production and suppression of iNOS and COX-2 at the mRNA and protein levels in LPS-stimulated RAW 264.7 cells [5] and in suppression of adipocyte differentiation in 3T3L-1 cells [30]. Structural change is one possible mechanism for anti-inflammation. In bioconversion of citrus with cytolase, rutinose and/or neohesperidose of flavanones were removed and these sugar groups were replaced with a hydroxyl group [15]. This addition of a free hydroxyl group might in part be responsible for suppression of pro-inflammatory cytokines and mediators in the CKP-C group. Although CKP and CKP-C contained almost equal amounts of flavanones (24.66% vs. 23.08%), it should be noted that CKP-C showed more potent activity than CKP. This may be ascribed in part to the main flavanone forms of CKP and CKP-C, which were hesperidin and hesperetin, respectively. As another example, it has been reported that hesperetin more effectively protected against oxidative stress than hesperidin in LLC-PK1 renal epithelial cells [31] and in rat cortical cells [32]. Therefore, bioconversion of CKP with cytolase is considered to boost more active compounds against pro-inflammation processes.
In conclusion, we evaluated the effects of the natural compound and its bioconverted compound on antioxidant and anti-inflammation. All of the citrus extracts showed DPPH radical scavenging activity and inhibited LPS-induced NO and PGE2 production by modulating the mRNA and/or protein expression of pro-inflammatory cytokines and mediators in LPS-stimulated RAW 264.7 cells. In particular CKP-C is superior in suppression of IL-1β, IL-6, and iNOS rather than TNF-α and COX-2. Taken together, bioconversion of citrus flavonoids with cytolase increased flavanone aglycosides, which may be a promising therapeutic agent for prevention of chronic diseases attributable to oxidation and inflammation by boosting the anti-inflammatory effects of citrus peels compared to flavanone glycosides.
Figures and Tables
 | Fig. 1Effects of CKP and CKP-C on cell viability.RAW 264.7 cells were treated with different concentrations (0-500 µg/ml) of CKP or CKP-C for 24 h. Cell viability was measured by MTT assay. Viability of untreated control cells was defined as 100%. CKP: Citrus kiyomi × ponkan peel extract; CKP-C: Citrus kiyomi × ponkan peel extract after bioconversion with cytolase. Each bar represents the mean ± SD (n = 3). Different letters mean significant difference according to ANOVA, Tukey test (P < 0.05).
|
 | Fig. 2Effects of CKP and CKP-C on DPPH free radical scavenging activity.Quercetin (50 µg/ml) was used as a positive control. Confluent cells were treated with 0-200 µg/ml of CKP and CKP-C and 0-50 µg/ml of quercetin for 4 h. CKP: Citrus kiyomi×ponkan peel extract; CKP-C: Citrus kiyomi × ponkan peel extract after bioconversion with cytolase. Each bar represents the mean ± SD (n = 3). Different letters mean significant difference according to ANOVA, Tukey test (P < 0.05).
|
 | Fig. 3Effects of CKP and CKP-C on nitric oxide (NO) and PGE2 production in LPS-stimulated RAW 264.7 cells.Cells were pre-treated with 0, 100, or 200 µg/ml of CKP or CKP-C for 4 h, followed by stimulation with 1 µg/ml of LPS for 8 h. The treated culture media were used to measure the production of NO and PGE2. CKP: Citrus kiyomi × ponkan peel extract; CKP-C: Citrus kiyomi × ponkan peel extract after bioconversion with cytolase. Each bar represents the mean ± SD (n = 3). Different letters mean significant difference according to ANOVA, Tukey test (P < 0.05).
|
 | Fig. 4Effects of CKP and CKP-C on mRNA levels of iNOS and COX-2 in LPS-stimulated RAW 264.7 cells.Cells were pre-treated with 0, 100, or 200 µg/ml of CKP or CKP-C for 4 h, followed by stimulation with 1 µg/ml of LPS for 8 h. Real time-PCR was performed using each cDNA and gene-specific primers. CKP: Citrus kiyomi × ponkan peel extract; CKP-C: Citrus kiyomi × ponkan peel extract after bioconversion with cytolase. Each bar represents the mean ± SD (n = 3). Different letters mean significant difference according to ANOVA, Tukey test (P < 0.05).
|
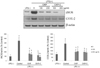 | Fig. 5Effects of CKP and CKP-C on protein expression of iNOS and COX-2 in LPS-stimulated RAW 264.7 cells.Cells were pre-treated with 0, 100, or 200 µg/ml of CKP or CKP-C for 4 h, followed by stimulation with 1 µg/ml of LPS for 8 h. Immunoblotting was performed with whole cell lysates and specific antibodies. CKP: Citrus kiyomi × ponkan peel extract; CKP-C: Citrus kiyomi × ponkan peel extract after bioconversion with cytolase; iNOS: inducible nitric oxide synthase; COX-2: cyclooxygenase-2.
|
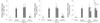 | Fig. 6Effects of CKP and CKP-C on mRNA levels of IL-1β, IL-6, and TNF-α in LPS-stimulated RAW 264.7 cells.Cells were pre-treated with 0, 100, or 200 µg/ml of CKP or CKP-C for 4 h, followed by stimulation with 1 µg/ml of LPS for 8 h. Real time-PCR was performed using each cDNA and gene-specific primers. CKP: Citrus kiyomi × ponkan peel extract; CKP-C: Citrus kiyomi × ponkan peel extract after bioconversion with cytolase. Each bar represents the mean ± SD (n = 3). Different letters mean significant difference according to ANOVA, Tukey test (P < 0.05).
|
References
1. Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000; 6:347–373.

3. Fan GW, Zhang Y, Jiang X, Zhu Y, Wang B, Su L, Cao W, Zhang H, Gao X. Anti-inflammatory activity of baicalein in LPS-stimulated RAW 264.7 macrophages via estrogen receptor and NF-κB-dependent pathways. Inflammation. 2013; 36:1584–1591.

4. Hu W, Yang X, Zhe C, Zhang Q, Sun L, Cao K. Puerarin inhibits iNOS, COX-2 and CRP expression via suppression of NF-κB activation in LPS-induced RAW 264.7 macrophage cells. Pharmacol Rep. 2011; 63:781–789.

5. Seo JE, Lim HJ, Chang YH, Park HR, Han BK, Jeong JK, Choi KS, Park SB, Choi HJ, Hwang JA. Effects of Jeju Citrus unshiu peel extracts before and after bioconversion with cytolase on anti-inflammatory activity in RAW 264.7 Cells. J Korean Soc Food Sci Nutr. 2015; 44:331–337.

6. Lee C, Oh H, Han S, Lim S. Effects of hot air and freeze drying methods on physicochemical properties of citrus 'Hallabong' powders. Food Sci Biotechnol. 2012; 21:1633–1639.

7. Treutter D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol (Stuttg). 2005; 7:581–591.

9. Bocco A, Cuvelier ME, Richard H, Berset C. Antioxidant activity and phenolic composition of citrus peel and seed extracts. J Agric Food Chem. 1998; 46:2123–2129.

10. Higashi-Okai K, Kamimoto K, Yoshioka A, Okai Y. Potent suppressive activity of fresh and dried peels from Satsuma mandarin Citrus unshiu (Marcorv.) on hydroperoxide generation from oxidized linoleic acid. Phytother Res. 2002; 16:781–784.

11. Suzuki M, Sasaki K, Yoshizaki F, Oguchi K, Fujisawa M, Cyong JC. Anti-hepatitis C virus effect of Citrus unshiu peel and its active ingredient nobiletin. Am J Chin Med. 2005; 33:87–94.

12. Jo CU, Park BJ, Chung SH, Kim CB, Cha BS, Byun MW. Antibacterial and anti-fungal activity of citrus (Citrus unshiu) essential oil extracted from peel by-products. Food Sci Biotechnol. 2004; 13:384–386.
13. Lee S, Ra J, Song JY, Gwak C, Kwon HJ, Yim SV, Hong SP, Kim J, Lee KH, Cho JJ, Park YS, Park CS, Ahn HJ. Extracts from Citrus unshiu promote immune-mediated inhibition of tumor growth in a murine renal cell carcinoma model. J Ethnopharmacol. 2011; 133:973–979.

14. Peterson J, Dwyer J. Flavonoids: dietary occurrence and biochemical activity. Nutr Res. 1998; 18:1995–2018.

15. Tripoli E, La Guardia M, Giammanco S, Di Majo D, Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 2007; 104:466–479.

16. Fuhr U, Kummert AL. The fate of naringin in humans: a key to grapefruit juice-drug interactions? Clin Pharmacol Ther. 1995; 58:365–373.

17. Seo JY, Lee JH, Kim NW, Her E, Chang SH, Ko NY, Yoo YH, Kim JW, Seo DW, Han JW, Kim YM, Choi WS. Effect of a fermented ginseng extract, BST204, on the expression of cyclooxygenase-2 in murine macrophages. Int Immunopharmacol. 2005; 5:929–936.

18. Yang HL, Chen SC, Senthil Kumar KJ, Yu KN, Lee Chao PD, Tsai SY, Hou YC, Hseu YC. Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: an ex vivo approach. J Agric Food Chem. 2012; 60:522–532.

19. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995; 28:25–30.

20. Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005; 81:230S–242S.

21. Marín L, Miguélez EM, Villar CJ, Lombó F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. BioMed Res Int. 2015; 2015:905215.

22. Bae EA, Han MJ, Kim DH. In vitro anti-Helicobacter pylori activity of some flavonoids and their metabolites. Planta Med. 1999; 65:442–443.

23. Kim HK, Jeon WK, Ko BS. Flavanone glycosides from Citrus junos and their anti-influenza virus activity. Planta Med. 2001; 67:548–549.

24. Chun SS, Vattem DA, Lin YT, Shetty K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem. 2005; 40:809–816.

25. Chao CL, Weng CS, Chang NC, Lin JS, Kao ST, Ho FM. Naringenin more effectively inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in macrophages than in microglia. Nutr Res. 2010; 30:858–864.

26. Blancke F, Claeys MJ, Jorens P, Vermeiren G, Bosmans J, Wuyts FL, Vrints CJ. Systemic inflammation and reperfusion injury in patients with acute myocardial infarction. Mediators Inflamm. 2005; 2005:385–389.

27. Kang K. Citrus peel extract inhibits LPS-induced cytokines secretion in macrophage. Int J Bio-Sci Bio-Tech. 2014; 6:11–20.

28. Kang SR, Han DY, Park KI, Park HS, Cho YB, Lee HJ, Lee WS, Ryu CH, Ha YL, Lee DH, Kim JA, Kim GS. Suppressive effect on lipopolysaccharide-induced proinflammatory mediators by Citrus aurantium L. in macrophage RAW 264.7 cells via NF-κB signal pathway. Evid Based Complement Alternat Med. 2011; 2011:248592.
29. Jung KH, Ha E, Kim MJ, Won HJ, Zheng LT, Kim HK, Hong SJ, Chung JH, Yim SV. Suppressive effects of nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) expression by Citrus reticulata extract in RAW 264.7 macrophage cells. Food Chem Toxicol. 2007; 45:1545–1550.

30. Lim H, Yeo E, Song E, Chang YH, Han BK, Choi HJ, Hwang J. Bioconversion of Citrus unshiu peel extracts with cytolase suppresses adipogenic activity in 3T3-L1 cells. Nutr Res Pract. 2015; 10:599–605.
31. Cho EJ, Li L, Yamabe N, Kim HY. Antioxidative effects of hesperidin and hesperetin under cellular system. CNU J Agric Sci. 2011; 38:717–722.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download