Abstract
The present study was performed to elucidate the hypocholesterolemic action of chitosan on the diet-induced hypercholesterolemia in rats. Male Sprague-Dawley rats (n=24) were fed with chitosan-free diet (Control), diets containing 2% or 5% chitosan for 4 weeks. Hypercholesterolemia was induced by adding 1% cholesterol and 0.5% cholic acid to all diets. Body weight gain and food intake of rats did not differ among the groups. The chitosan treated groups showed significant improvement in the plasma concentration of total cholesterol and LDL-cholesterol compared to the control group (p<0.05). Also, the chitosan treated groups decreased the liver concentration of total lipid and total cholesterol compared to the control group (p<0.05). The activity of hepatic cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme in the conversion of cholesterol to bile acids, was increased by 123% and 165% for the 2% or 5% chitosan diets, respectively. These findings suggest that enhancement of hepatic CYP7A1 activity may be a mechanism, which can partially account for the hypocholesterolemic effect of dietary chitosan in cholesterol metabolism.
An increased blood cholesterol level is one of the major risk factors for the development of coronary heart disease (CHD) (Assmann et al., 1999). Risk factors for CHD include aging, hypercholesterolemia, hypertension and hyperlipidemia (Castelli et al., 1986). A high blood cholesterol level is ranked as one of the greatest risk factors contributing to the prevalence and severity of CHD (Grundy, 1986; Neaton et al., 1984). The blood cholesterol level is determined by the balance among the dietary cholesterol, cholesterol biosynthesis and conversion of cholesterol to bile acids in the liver, and cholesterol absorption and reuptake by the small intestine (Assmann et al., 1999). Cholesterol 7α-hydroxylase (CYP7A1) is a liver-specific cytochrome P450 isozyme of the CYP7A family that catalyzes the rate-limiting step in the classic pathway of bile acid biosynthesis. Conversion of cholesterol to bile acids in the liver is the most important pathway for the elimination of cholesterol from the body (Turley & Dietschy, 1988). Transcription of CYP7A1 is inhibited in a feedback mechanism by bile acids and stimulated in a feed-forward mechanism by cholesterol (Guorong et al., 2004; Spady et al., 1996).
Chitosan is obtained by the deacetylation of chitin, an aminopolysaccharide found in the exoskeletons and the fungal cell wall of various arthropods including insects, crabs and shrimps (Muzzarelli, 1977). Although it is not derived from plants, it shares the same characteristics as dietary fiber, which is a polysaccharide indigestible by mammalian digestive enzymes. When ingested, chitosan develops an HCl-layer in the stomach. As capsulated particles of chitosan move into the duodenum, the HCl-layer becomes diluted and the chitosan particles form agglomerates with fatty acids and cholesterol, thus reducing lipid absorption from the gastrointestinal tract. Studies in primates have shown that chitosan can increase the amount of fat eliminated in the stool (Ebihara et al., 1989; Sugano et al., 1980).
Several studies showed that chitosan had a potent plasma lipid lowering effect in the rat (Hossain et al., 2007; Sugano et al., 1980; Xu et al., 2007) and human (Bokura & Kobayashi, 2003; Gallaher et al., 2002; Guha et al., 2005). Daily fecal bile acid excretion in the 7.5% chitosan group was more than twofold greater than that of the control and glucomannan groups (Gallaher et al., 2000). Maezaki et al. (1993) reported increased fecal excretion of two bile acids, cholic and chenodeoxycholic acid in male subjects consuming 3 to 6 g/day of chitosan. In rats, chitosan increased (Sugano et al., 1980) or had no effect (Fukada et al., 1991) in fecal neutral sterol excretion. The 3-hydroxy-3-methyl glutaryl-CoA (HMG-CoA) reductase activity was elevated in chitosan group although it was lower than that of the cholesterol free group, whereas the HMG-CoA reductase mRNA levels were not changed (LeHoux & Grondin, 1993).
Investigations of the hypocholesterolemic effect of chitosan have focused on greater excretion of bile acids and total steroids leading to the increase of bile acid biosynthesis. Synthesis of bile acids from cholesterol is regulated by feedback inhibition of the rate-limiting enzyme, cholesterol 7α-hydroxylase (CYP-7A1) (Danielsson et al., 1975). However, the effect of chitosan on the activity of liver CYP7A1 has not been described.
Therefore, the purpose of this study was to investigate the effects of chitosan on plasma and liver cholesterol levels in cholesterol-fed rats. Additionally, we determined whether chitosan would regulate the activity of the hepatic CYP7A1 in cholesterol-fed rats.
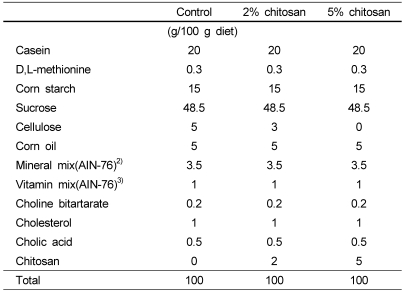
Three groups of male Sprague-Dawley rats (initial weights, 130 ± 5 g, SLC, Japan) were housed individually in a light controlled room (dark, 06:00-18:00 hour) at a temperature of 22 ± 2℃ and relative humidity 55 ± 5%. Rats were given free access to a non-purified diet (Ralston Purina, St Louis, MO) and tap water for 1 week to be acclimatized before the experiment. Rats were randomly divided into three groups and assigned to different dietary treatments. The compositions of the experimental diets are shown in Table 1. Experimental diets employed were modifications of the AIN-76 purified rodent diet (Dyets, Bethlehem, PA). Rats were fed chitosan-free diets (control), diets with the addition of 2% or 5% chitosan and tap water ad libitum for 4 weeks. Chitosan from sea crab shell was obtained from Iljin Pharm Co. Ltd., Korea. All diets contained 1% cholesterol and 0.5% cholic acid in order to induce hypercholesterolemia. The Rats were given free access to food and water for 4 weeks, and food intake and body weight gain were monitored twice a week. At the end of the experiment, rats were anesthetized and a central longitudinal incision was made into the abdominal wall, and blood samples were collected by cardiac puncture. Blood samples were centrifuged at 1,500 × g for 20 minutes at 4℃ and the plasma was separated and stored at -20℃ until analyzed. Liver samples were excised, immediately frozen in liquid nitrogen and stored at -70℃. All animal procedures conformed to NIH guidelines as stated in the "Principles of Laboratory Animal Care" published by the National Institutes of Health (publication No. 86-23, revised 1985).
The plasma total cholesterol, HDL-cholesterol and triglyceride levels were determined by enzymatic colorimetric methods using commercial kits (Asan Pharmaceutical, Seoul, Korea). Plasma LDL-cholesterol was calculated by the formula of Friedewald et al. (1972). Total lipids in the liver were extracted with a chloroform: methanol mixture (2:1, v/v) as described by Folch et al.(1956). The concentration of liver cholesterol in the lipid extracts was measured enzymatically by using a kit (Asan Pharmaceutical, Seoul, Korea).
CYP7A1 activity was assayed using the procedure of Oda et al. (1989). About 2 g of liver was homogenized in 4 volumes of 0.1 M potassium phosphate buffer (pH 7.4) containing 1 mM EDTA and 50 mM NaF. The homogenates were centrifuged at 12,000 × g for 15 minutes, and the supernatant was re-centrifuged at 100,000 × g for 60 minutes. The microsomal pellet was suspended in 2 ml of 0.1 M potassium phosphate buffer (pH 7.4) containing 0.1 mM EDTA, 50 mM NaF, 2 mM NADPH, 20 mM cysteamine, 200 µM cholesterol, 1.5 g Tween 80, and 6 µCi [7(n)-3H] cholesterol (Amersham, UK). Incubation was carried out at 37℃ for 30 minutes. The reaction was stopped by the addition of 6 ml of 20% trichloroacetic acid. Subsequently, the reaction mixture was centrifuged at 1,500 × g for 10 minutes. Chloroform was added to the supernatant to extract the radiolabeled cholesterol. After the second extraction with chloroform, 1 ml of the upper aqueous phase was transferred, and the radioactivity was quantified with a liquid scintillation counter. The protein content in microsomes was measured according to Bradford (1976).
Data are expressed as mean ± SD. The significant differences among groups were determined by one-way analysis of variance using the SPSS package program, version 11.0 (SPSS, Chicago, IL, USA). The results were considered significant if the value of p was <0.05, and Duncan's multiple range test was performed if differences were identified between groups.
Body weight gain, food intake and food efficiency ratio (FER) of rats did not differ among the groups, in spite of chitosan supplementation (Table 2). Rats treated with 2% or 5% chitosan had significantly lower plasma total cholesterol concentrations by 17% and 30%, respectively, than that of rats fed the control diet. Also, the 2% or 5% chitosan groups significantly decreased plasma LDL-cholesterol concentrations by 28% and 41%, respectively, while the plasma HDL-cholesterol and triglyceride concentrations were not significantly affected. The atherogenic index (AI) was decreased by chitosan supplementation. In addition, total lipid and total cholesterol in liver were significantly decreased by 57% and 40%, respectively, in 5% chitosan diet compared to the control group (Table 2).
Consumption of chitosan diet resulted in the elevated activity of hepatic CYP7A1 compared to the control group. The 2% or 5% chitosan diet increased the activity of CYP7A1 by 123% and 165%, respectively, compared to the control group (Fig. 1).
Our study was carried out in order to clarify how chitosan affects the cholesterol metabolism in hypercholesterolemic rats. The results showed that concentrations of plasma cholesterol in rats fed chitosan were reduced. In this study, we added 2% or 5% chitosan to diet containing 1% cholesterol and 0.5% cholic acid and treated rats for 4 weeks. Consumption of 2% or 5% chitosan did not suppress body weight gain, food intake and food efficiency compared to the control group. Similar to our results, Sugano et al. (1980) reported that intake of 2% or 5% chitosan was not significantly different in body weight gain and food intake.
After 4 weeks of experimental period, chitosan diets showed positive results showing reduction in the plasma total cholesterol, LDL-cholesterol and atherogenic index in diet-induced hypercholesterolemic rats. The hypocholesterolemic effects of chitosan diets tested were as follows: chitosan 5% > chitosan 2% > control. Razdan et al. (1994) showed that feeding of chitosan-containing diets generally reduced the plasma total cholesterol and HDL cholesterol concentrations and gave an increased HDL to total cholesterol ratio in comparison with chickens given the control and chitin-containing diet. However, no significant reductions in the plasma triacylglyceride concentrations resulted from feeding of the chitosan-containing diets were observed.
To evaluate effects of dietary fiber on the cholesterol metabolism, the dietary cholesterol concentrations usually employed were ranged from 0.2% to 1%. Our diet included 1% cholesterol and 0.5% cholic acid. The ability of chitosan to improve cholesterol metabolism might have several explanations. Addition of 2, 5 or 10% chitosan resulted in a significant reduction in the plasma cholesterol, liver cholesterol and triacylglyceride but 10% level of chitosan depressed the animal growth (Sugano et al., 1980). These results implied that high dose (more than 10%) of chitosan might be toxic to the rat. The most commonly reported adverse effects of chitosan are gastrointestinal problems, such as nausea, diarrhea and constipation. These effects generally do not cause discontinuation of chitosan use (Hendler et al., 2001).
Chitosan treatment decreased the liver total lipid and total cholesterol contents compared to the control diet. Similar to our results, Chiang et al. (2000) reported that intake of 5% high viscosity chitosan and 5% low viscosity chitosan decreased the liver total lipids and total cholesterol concentrations compared to the control group.
Chitosan has been shown to be hypocholesterolemic based on the mechanism of greater excretion of bile acids and total steroids leading to an up-regulation of bile acid biosynthesis (Fukada et al., 1991; Gallaher et al., 2000; Maezaki et al., 1993; Murata et al., 2006). However, the effects of chitosan on the bile acid synthesizing enzyme, CYP7A1, have not been investigated.
In our results, supplementation of chitosan increased the activity of hepatic microsomal CYP7A1. A plausible mechanism for the hypocholesterolemic activity of chitosan is its up-regulation of fecal neutral acids excretion. Conversion of cholesterol to neutral acids is the major pathway of cholesterol elimination (Kelly, 2003). The chitosan in the diet may lead to a diminished absorption of bile acids, thereby resulting in up-regulation of hepatic CYP7A1 activity. By feeding chitosan to rats, the CYP7A1 activity was stimulated compared to the control, resulting in the hypocholesterolemic effect. Our finding indicated that chitosan may possess hypocholesterolemic actions, which operate in a manner dependent of CYP7A1 activity.
In conclusion, the addition of chitosan to rats had a beneficial effect on the cholesterol metabolism. Chitosan treatment enhanced the hepatic CYP7A1 activity, which was associated with the decrease in the plasma total cholesterol level. Increase in the CYP7A1 activity may play a role in the hypocholesterolemic action of chitosan in vivo. Thus, the consumption of chitosan may be considerably beneficial to hypercholesterolemic patients. Further research is required to fully delineate the mechanisms that contribute to the hypocholesterolemic effects of chitosan and its constituents.
References
1. Assmann G, Cullen P, Jossa F, Lewis B, Mancini M. Coronary heart disease: reducing the risk: the scientific background to primary and secondary prevention of coronary heart disease. A worldwide view. International task force for the prevention of coronary heart disease. Arterioscler Thromb Vasc Biol. 1999; 19:1819–1824. PMID: 10446059.
2. Bokura H, Kobayashi S. Chitosan decreases total cholesterol in women: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2003; 57:721–725. PMID: 12771974.

3. Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254. PMID: 942051.

4. Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The framingham study. JAMA. 1986; 256:2835–2838. PMID: 3773200.

5. Chiang MT, Yao HT, Chen HC. Effect of dietary chitosans with different viscosity on plasma lipids and lipid peroxidation in rats fed on a diet enriched with cholesterol. Biosci Biotechnol Biochem. 2000; 64:965–971. PMID: 10879465.

6. Danielsson H, Sjovall J. Bile acid metabolism. Annu Rev Biochem. 1975; 44:233–253. PMID: 1094911.

7. Ebihara K, Schneeman BO. Interaction of bile acids, phospholipids, cholesterol and triglyceride with dietary fibers in the small intestine of rats. J Nutr. 1989; 119:1100–1106. PMID: 2550597.

8. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957; 226:497–509. PMID: 13428781.
9. Friedewald WT, Levey RI. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502. PMID: 4337382.

10. Fukada Y, Kimura K, Ayaki Y. Effect of chitosan feeding on intestinal bile acid metabolism in rats. Lipids. 1991; 26:395–399. PMID: 1895888.

11. Gallaher CM, Munion J, Hesslink R Jr, Wise J, Gallaher DD. Cholesterol reduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats. J Nutr. 2000; 130:2753–2759. PMID: 11053517.

12. Gallaher DD, Gallaher CM, Mahrt GJ, Carr TP, Hollingshead CH, Hesslink R Jr, Wise J. A Glucomannan and Chitosan Fiber Supplement Decreases Plasma Cholesterol and increases cholesterol excretion in overweight normo-cholesterolemic Humans. J Am Coll Nutr. 2002; 21:428–433. PMID: 12356785.

13. Grundy SM. Cholesterol and coronary heart disease: a new era. JAMA. 1986; 256:2849–2858. PMID: 3534335.

14. Guha S, Pal SK, Chatterjee N, Sarkar G, Pal S, Guha S, Basu AK, Banerjee R. Effect of chitosan on lipid levels when administered concurrently with atorvastatin-a placebo controlled study. J Indian Med Assoc. 2005; 103:418–420. PMID: 16363196.
15. Guorong X, Lu-xing P, Hai L, Quan S, Akira H, Sarah S, Jaya B, Yasushi MG, Stephen T, Gerald S. Dietary cholesterol stimulates CYP7A1 in rats because farnesoid X receptor is notactivated. Am J Physiol Gastrointest Liver physiol. 2004; 286:G730–G735. PMID: 14684380.
16. Hendler SS, Rorvik D. PDR for nutritional supplements. 2001. New York. USA: Thomson Healthcare;Montvale NJ: medical ecomomics.
17. Hossain S, Rahman A, Kabir Y, Shams AA, Afros F, Hashimoto M. Effects of shrimp (Macrobracium rosenbergii)-derived chitosan on plasma lipid profile and liver lipid peroxide levels in normo- and hypercholesterolaemic rats. Clin Exp Pharmacol Physiol. 2007; 34:170–176. PMID: 17250635.

18. Kelly M. Chitosan for weight loss and cholesterol management. Am J health syst pharm. 2003; 60:1310–1315. PMID: 12901030.
19. LeHoux JG, Grondin F. Some effects of chitosan on liver function in the rat. Endocrinology. 1993; 132:1078–1084. PMID: 7679967.

20. Maezaki Y, Tsuji K, Nakagawa Y, Kawai Y, Akimoto M. Hypocholesterolemic effect of chitosan in adult males. Biosci Biotech Biochem. 1993; 57:1439–1444.

21. Murata Y, Nagaki K, Kofuji K, Sanae F, Kontani H, Kawashima S. Adsorption of bile acid by chitosan salts prepared with cinnamic acid and analogue compounds. J Biomater Sci Polym Ed. 2006; 17:781–789. PMID: 16909945.

22. Muzzarelli RAA. Enzymatic synthesis of chitin and chitosan. Occurrence of chitin. Chitin. 1977. New York. USA: Pergamon Press;p. 5–17.
23. National Research Council. Publication No. 85-123. Guide for the care and use of laboratory animals. 1985. Bethesda, MD. USA: National Institutes of Health.
24. Neaton JD, Kuller LH, Wentworth D, Borhani NO. Total and cardiovascular mortality in relation to cigarette smoking, plasma cholesterol concentration and diastolic blood pressure among black and white males followed up for five years. Am Heart J. 1984; 108:759–769. PMID: 6475745.
25. Oda H, Okumura Y, Hitomi Y, Ozaki K, Nagaoka S, Yoshida A. Effect of dietary methionine and polychlorinated biphenyls on cholesterol metabolism in rats fed a diet containing soy protein isolate. J Nutr Sci Vitaminol. 1989; 35:333–348. PMID: 2511287.

26. Spady DK, Cuthbert JA, Willard MN, Meidall RS. Feedback regulation of hepatic 7α-hydroxylase expression by bile salts in the hamster. J Biol Chem. 1996; 271:18623–18631. PMID: 8702514.

27. Sugano M, Fujikawa T, Hiratsuji Y, Nakashima K, Furada N, Hasegawa Y. A novel use of chitosan as a hypocholesterolemic agent in rats. Am J Clin Nutr. 1980; 33:787–793. PMID: 7361697.

28. Turley SD, Dietschy JM. The metabolism and excretion of cholesterol by the liver. The liver: Biology and Pathobiology. 1988. New York. USA: Raven Press;p. 617–639.
29. Xu G, Huang X, Qiu L, Wu J, Hu Y. Mechanism study of chitosan on lipid metabolism in hyperlipidemic rats. Asia Pac J Clin Nutr. 2007; 16:313–317. PMID: 17392126.
Fig. 1
Effect of dietary chitosan on hepatic CYP7A1 activity in cholesterol-fed rats. Values are expressed as mean ± SD, n = 8. Different superscripts are significantly different (p<0.05).
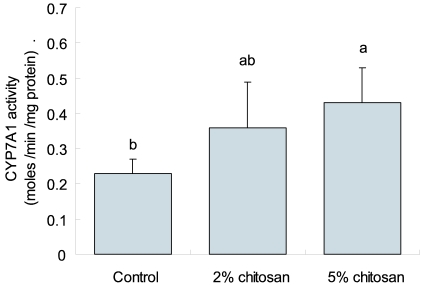
Table 2
Body weight gain, food intake, FER and lipid profiles of rats1,2)
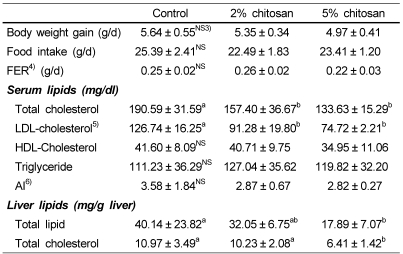
1)Values are expressed as mean ± SD (n=8).
2)Values in a column with different superscripts are significantly different, p<0.05.
3)NS is not significant.
4)Food efficiency ratio (FER)=body weight gain (g/d)/food intake (g/d).
5)LDL cholesterol was calculated by the method of Friedewald WT formula.
6)AI (Atherogenic Index)=(Total Cholesterol-HDL-Cholesterol)/HDL-Cholesterol.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download