Abstract
Folate has received international attention regarding its role in the risk-reduction of birth defects, specifically neural tube defects (NTDs). In 1998, health officials in Canada, like the United States, mandated the addition of folic acid to white flour and select grain products to increase the folate intake of reproductive-aged women. Subsequent to this initiative there has been an increase in blood folate concentrations in Canada and a 50% reduction in NTDs. Many countries, including Korea, have not mandated folic acid fortification of their food supply. Reasons vary but often include concern over the masking of vitamin B12 deficiency, a belief that folate intakes among womenare adequate, low priority relative to other domestic issues, and the philosophy that individuals have the right not to consume supplemental folic acid if they so choose. Prior to folic acid fortification of the food supply in Canada, the folate intakes of women were low, and their blood folate concentrations while not sufficiently low to produce overt signs of folate deficiency (eg. anemia) were inconsistent with a level known to reduce the risk of an NTD-affected pregnancy. The purpose of this article is to describe the role of folate during the periconceptional period, pregnancy, and during lactation. The rationale for, and history of recommending folic acid-containing supplements during the periconceptional period and pregnancy is described as is folic acid fortification of the food supply. The impact of folic acid fortification in Canada is discussed, and unresolved issues associated with this policy described. While the incidence of NTDs in Canada pre-folic acid fortification were seemingly higherthan that of Korea today, blood folate levels of Korean women are strikingly similar. We will briefly explore these parallels in an attempt to understand whether folic acid fortification of the food supply in Korea might be worth consideration
Folate, a water-soluble B vitamin, is a generic descriptor for a host of structurally similar compounds that contain a pteroylmonoglutamic acid or a folic acid core (Fig. 1). This consists of a p-aminobenzoic acid molecule linked to a pteridine ring at one end and glutamic acid at the other. Pteroylmonoglutamic acid or folic acid, is seldom found in nature, but because of its stability and low cost, is synthesized and used in vitamin supplements and as a fortificant in food. By convention, the term folic acid is typically reserved to describe the synthetic form of the vitamin. Folates function in many coenzyme forms in acceptance, redox processing and transfer of one-carbon units. They are best known for their key role in the synthesis and repair of DNA, and methylation of homocysteine to regenerate methionine (Fig. 2).
Inadequate folate intakes have been associated in the literature with a number of negative health outcomes in humans including neural tube defects (NTDs), cleft lip and/or palate, low infant birth weight, abruptio placenta, preeclampsia, spontaneous abortion, stillbirth, macrocytic anemia, cardiovascular disease and neuropsychiatric disorders (Lucock, 2000; Picciano et al., 1994; Ray, 1998; Seshadri et al., 2002; Tamura & Picciano, 2006). Suboptimal intakes have also been shown in some studies to play a role in the development of several malignancies including cancer of the colorectum, breast, cervix, lung, pancreas, esophagus, and stomach as well as neuroblastoma and leukemia (French et al., 2003; Kim, 1999; Kim, 2003; Kim, 2004b) Canadian Health authorities concluded that the evidence in support of NTD prevention was sufficiently robust that it mandated folic acid fortification of white flour and select grain products as of November 1998. Many countries globally, however, have not adopted a folic acid fortification policy. Many have not done so because of concern over the potential adverse effects of folic acid, in particular the concern that high intakes of folic acid may delay the diagnosis of B12 deficiency by correcting the characteristic megaloblastic anemia.
The purpose of this article is to describe the role of folate during female reproduction and specifically the periconceptional period, pregnancy (post-closure of the neural tube), and during lactation. The rationale for and history of recommending folic acid-containing supplements during the periconceptional period and pregnancy in Canada is described as is the folic acid fortification policy. The impact of folic acid fortification is discussed and unresolved and immerging issues associated with this policy are described. While the incidence of NTDs pre-folic acid fortification of the food supply in Canada were higher than that of South Korea today, blood folate levels of Korean women are strikingly similar. We will briefly explore these parallels in an attempt to understand whether folic acid fortification of the food supply in South Korea might be worth consideration.
As described above, folates function in many coenzyme forms in acceptance, redox processing and transfer of one-carbon units. In order to carry out these functions, folates in nature are typically reduced to either di-hydro or tetrahydrofolate forms with hydrogens at the 5, 6, 7 and 8 positions (Fig. 1). Further, various one-carbon units can be carried at the N5 and N10 positions or bridging the same. Finally, in nature, a significant proportion of folates are polyglutamylated, meaning they have several glutamates linked together to create what is commonly referred to as a polyglutamate tail.
Embryonic, fetal and infant growth occurs more rapidly than at any other stage of the life cycle. The anabolic activity that must occur during pregnancy and lactation to support this growth, and requisite DNA, RNA and amino acid biosynthesis dictates an elevated dietary requirement for folate. Folate, in the form of 5,10-methylene tetrahydrofolate, during DNA synthesis acts as a methyl donor for the enzyme thymidylate synthase which converts deoxyuridine monophosphate to thymidine monophosphate (Fig. 2). Folate in the form of 10-formyl tetrahydrofolate is necessary for the synthesis of the purines adenine and guanine, the nucleic acid building blocks of DNA and RNA. The amino acids methionine, serine, glycine and histidine are likewise metabolized via folate-dependent reactions.
Folate in the form of 5-methyltetrahydrofolate, is involved in remethylation of homocysteine to methionine. The latter is a precursor for S-adenosylmethionine (SAM), the principle methyl group donor in the body. SAM is involved in methylating cytosine in DNA and is thought to play a key role in post-transcription regulation of gene expression. Myelin maintenance and neural function are likewise dependent on the methylation reactions involving SAM.
Folates are synthesized in plants and certain bacteria but not in mammalian cells, and thus, they must be obtained in the diet. The recommended dietary allowance (RDA=dietary intake sufficient to meet the requirements of 97-98% of healthy individuals) of folate for adults, is 400µg/day of dietary folate equivalents (DFEs) (Institute of Medicine, 1998). The concept of DFEs for folate was introduced in North America in 2000 to account for the differences in bioavailability of synthetic folic acid and naturally occurring folates (Institute of Medicine, 1998). Compared to folic acid consumed alone (a relative availability of 100%), folic acid ingested with food or used as a fortificant is thought to be only ~85% available. In contrast, naturally occurring food folates are thought to be only ~50% available (Institute of Medicine, 1998; Pfeiffer et al., 1997). Thus, folic acid is calculated to be 1.7 (85 divided by 50) more available than naturally occurring food folates. Hence, in order to convert all forms of dietary folate into DFEs, the following calculation was developed: µg of DFEs provided = µg food folate + (µg folic acid × 1.7). The Estimated Average Requirement (EAR = dietary intake that meets the biologic requirement for 50% of the population) and the RDA are comparable for men and women, yet they differ when compared to the recommendations for pregnant and lactating women (Institute of Medicine, 1998). The RDA for pregnant and lactating women is 600 and 500 µg/day DFE, respectively, compared to 400 µg/day for nonpregnant nonlactating women (Institute of Medicine, 1998; Table 1).
A fetus' spinal cord and brain develop from the neural tube, which forms between seventeen and thirty days after conception, often before most women realize they are pregnant (Institute of Medicine, 1998). Spina bifida and anencephaly, the two most common neural tube defects, occur when the neural tube does not close properly. Spina bifida is a congenital defect in the spinal column, characterized by the absence of the vertebral arches through which the spinal membranes and spinal cord may protrude. Anencephaly, on the other hand, is characterized by an absence of the brain and cranial vault, with the cerebral hemispheres completely missing or greatly reduced in size.
A relationship between NTDs and sub-optimal folate status was first proposed over 30 years ago by Smithells et al. (1976). Following a series of aggressively challenged cohort, case control, non-randomized control studies and a small randomized control trial, two major randomized clinical control trials: one co-ordinated by the UK Medical Research Council and the other conducted in conjunction with the Hungarian Family Planning program produced a flurry of public policy activity in North America regarding the use of folic acid during the periconceptional period (Czeizel, 1993; MRC Vitamin Study Research Group, 1991; Picciano et al., 1994). The UK Medical Research Council Study (1991) was an international multicentre (33 centres) randomized double blind prevention trial (n=1,817) which demonstrated that supplementation with 4 mg/day of folic acid during the periconceptional period resulted in a three-fold reduction in NTDs among women who had experienced a previous NTD-affected pregnancy (MRC Vitamin Study Research Group, 1991). Specifically in this high risk population, the recurrence rate was only 5 in 593 women who received a folic acid supplement and 21 of 602 who did not. In the Hungarian study, women (n=4,156) without a previous NTD-affected pregnancy and planning to become pregnant again were randomly assigned to receive a multivitamin-mineral supplement (including 0.8mg of folic acid) or a placebo (no folic acid but contained copper, manganese, zinc and vitamin C) (Czeizel, 1993). Participants of this study were defined as fully compliant if they took the supplements for at least 28 days before conception and continued taking them until the date of the second missed menstrual period. Six cases of NTDs were found in the placebo group (n=2,104); whereas, no NTDs were reported among periconceptional multivitamin-mineral supplement users (n=2,052). These results suggest that for every 342 women treated with a folate-containing multivitamin-mineral supplement, one NTD would be prevented among women without a previous history of a NTD affected pregnancy.
The mechanism(s) how supplemental folic acid reduces the risk of a NTD is unclear; however as described by Blom et al. (2006), it may involve folate's role in methylation of DNA-"the methylation hypothesis". Briefly this hypothesis suggests that the enzyme methylene tetrahydrofolate reductase (MTHFR) sits at an important juncture in folate metabolism and depending on its activity may preferentially shunt one-carbon units toward either (1) methylation (production of SAM) or (2) thymidine and purine synthesis (production of DNA and RNA) (Fig. 2). Identification of a common single nucleotide polymorphism (SNP) in the MTHFR gene (677C >T) and our understanding of how this polymorphism may shunt folate in the direction of DNA and RNA biosynthesis versus SAM is consistent with the "methylation" hypothesis. Likewise, observations of reduced global DNA methylation and an increased NTD risk among carriers of the MTHFR 677C >T polymorphism in some, but not all studies, adds further credibility to the "methylation" hypothesis. In their meta-analysis of available studies, Blom et al report that women homozygous for the 677C >T MTHFR variant (677TT) have a 60% increased risk of an NTD-affected pregnancy.
While few in number, studies are available in the literature that link maternal folate status with other aberrations in early embryonic development. Such investigations include examination of the role of folate in congenital heart defects, urinary tract anomalies, limb defects and in unexplained recurrent early pregnancy loss to name a few. It is beyond the scope of this review to discuss each in detail but it is fair to say that while data from these studies are very interesting for most associations, the body of evidence is insufficient to make solid conclusions about them at this time (Botto et al., 1996; Czeizel, 1993; Czeizel, 1996; Li et al., 1995; Shaw et al., 1995; Wouters et al., 1993). The reader is directed to the comprehensive review recently published by Tamura & Picciano (2006) for a systematic discussion of this literature. Two congenital anomalies where there has been a significant body of work completed to link their origin to sub-optimal maternal folate status are orofacial defects (e.g. cleft lip and cleft palate) and Downs Syndrome.
Due to the success in preventing NTDs with folic acid supplementation (Tamura & Picciano, 2006), and speculation that orofacial structure and neurocrest closure may be linked, researchers have examined whether maternal folate nutrition and/or folic acid supplementation during the periconceptional period may have an impact on orofacial clefts. The critical period for fetal lip and palate formation is between 6 and 12 week of gestation. Tolarova (1982) reported a reduced re-occurrence (randomized control trial), and Czeizel et al. (1999) a reduced occurrence (prospective cohort study) of orofacial clefts in infants born to women supplemented with pharmacologic levels of folic acid (6-10 mg/d) during the periconceptional period. Recently Wilcox et al. (2007) reported on a national population based case-control from Norway (n=377 infants with cleft lip with or without cleft palate; 196 infants with cleft palate alone; 763 normal controls) in which folic acid supplements (>400 ug/d) during early pregnancy reduced the risk of isolated cleft lip (with or without cleft palate) by about a third. There was no effect of folic acid supplementation on cleft palate alone. Badovinac et al. (2007) in a meta-analysis of all published studies in English reported a protective effect of any level of folic acid supplementation during pregnancy on oral clefts. In contrast, Ray et al. (2003) reported no change in the risk for orofacial clefts preand post-folic acid fortification of the food supply in Canada. There is epidemiological evidence that the offspring of mothers' homozygous for the 677C >T MTHFR variant may be at increased risk of orofacial clefts, especially among women with low folate intake in some (Mills et al., 1999; van Rooij et al., 2003), but not all studies (Jugessur et al., 2003; Shaw et al., 1998)
In addition to the etiologic role of folate in NTDs and orofacial clefts, investigation of the relationship between maternal folate nutrition and frequency of Down syndrome has been an active area of research. Down Syndrome, or trisomy 21, results from an extra copy of chromosome 21, 95% of which is of maternal origin (Eskes, 2006). An increased prevalence of the 677C >T SNPs in the MTHFR gene among mothers who gave birth to a child with Down syndrome has been observed in many but not all populations examined (Eskes, 2006). Similarly it has been observed that the frequency of a methionine synthase reductase gene varient (A66A>G) is higher among mothers of children with Down syndrome than without and that the MTHFR 677C >T and methionine synthase A66A>G variants in combination in women may be predictive of an infant with Down syndrome. As with MTHFR, methionine synthase is a key enzyme in folate metabolism (Fig. 2).
Prior to the commencement of population-based initiatives in Canada to reduce the risk of folate-dependent NTDs (before 1990), the primary consideration of public policy makers in relation to folate was to prevent the precipitous decline in maternal folate status that typically occurred in the 2nd and 3rd trimesters of pregnancy. The fact that the ONLY vitamin and/or mineral supplement recommended for adults by Health Canada, at any physiological state, was one for folic acid during pregnancy, underscores the level of concern regarding this phenomenon.
Indeed, folate requirements are higher during anabolic periods of the life cycle including pregnancy and lactation, and in utero. Chanarin, followed by a host of others in the 1970's, confirmed that pregnancy increased the requirement for folate which was not generally being met by a con-commitant increase in dietary folate intake (Chanarin, 1969). Folate requirements increase during pregnancy with an expansion of blood volume (~49%) and increased cellular proliferation as a result of uterine enlargement, placental development and fetal growth (Blackburn & Loper, 1992; Picciano et al., 1994). Prior to 1992 in Canada, women at their first prenatal visit (10-12 weeks after conception), but not before, were advised to take a folate-containing multivitamin mineral prenatal supplement (0.8-1.0 mg/d) for the duration of pregnancy. This supplement served to curb the decline in maternal blood folate concentrations during pregnancy and addressed possible clinical sequelae such as maternal megaloblastic anemia, placental abruption, pre-eclampsia, spontaneous abortion, stillbirth, poor fetal growth and premature delivery. A brief discussion of the evidence in support of the protective role of adequate maternal folate nutrition to promote normal fetal growth and prevention of premature delivery is provided below. The reader is once again referred to the review article by Tamura and Piccano (2006) for a detailed discussion of the weight of the evidence in support other clinical sequelae of suboptimal maternal folate nutrition.
Folate supplementation of pregnant women in a number of developing countries, where maternal dietary intake of folate are low, has been shown to increase infant birth weight and length, possibly mediated by improved placental development (Baumslag et al., 1970; Iyengar & Rajalakshmi, 1975). Likewise an association between maternal folate status and birth weight has been observed in the very economically and socially disadvantaged community of Camden New Jersey in the United States. Scholl et al reported that among 832 low-income women from Camden, those with mean dietary folate intakes below 240 µg/d had an approximate two-fold greater risk of preterm delivery and low infant birth weight than women with higher folate intakes (Scholl et al., 1996). The relationship between maternal folate nutritional status and birth weight in developed countries is not as consistent as observed in developing countries, likely reflecting a generally better overall plane of maternal folate nutrition. Interestingly, Vollset et al (Vollset et al., 2000) reported that women (n=5,883) in the upper quartile of plasma homocysteine concentration had an increased risk for delivering an infant prematurely (38%) or of low infant birth weight (101%) as recorded in the Medical Birth Registry of Norway compared to women in the lowest quartile of plasma homocysteine concentration (p<0.005). Given that birth weight is probably the most significant predictor of infant mortality and morbidity in the first year of life, these observations have important public health implications (Wilcox, 2001).
All of the aforementioned studies examining the relationship between maternal folate status and clinical sequelae were conducted prior to folic acid fortification of the food supply in North America or were carried out in countries where folic acid fortification has not been initiated. As will be discussed in greater detail later in this review, mandatory folic acid fortification in North America has virtually eliminated folate deficiency among healthy, non-reproducing adults. This raises the question of whether, or not, post-folic acid fortification one would expect a decline in maternal folate status during pregnancy in the absence of a folic acid-containing supplement.
Breastfeeding is the gold standard and strongly preferred method of feeding infants. The World Health Organization and Health Canada recommends human milk as the exclusive nutrient source for full-term infants for the first 6 month of life and indicates that breastfeeding be continued at least through the first 12 months of life and thereafter as long as mother and baby mutually desire (Health Canada, 2004; WHO, 2001). The scientific rationale for recommending breastfeeding as the preferred feeding choice for infants stems from its acknowledged benefits to infant nutrition, gastrointestinal function, host defense, neurodevelopment and psychological, economic and environmental well-being (American Academy of Pediatrics, 2005; Kleinman, 2004). In contrast to that of pregnancy, research regarding the folate requirements of women during lactation has received very little attention. What does appear clear from the literature, however, is that milk folate levels are maintained at the expense of maternal folate reserves, except in the most severe cases of maternal folate deficiency (e.g. megaloblastic anemia). Hence, under usual circumstances the nursing infant is protected from maternal folate inadequacy (O'Connor et al., 1997). Milk folate concentrations in the literature do vary considerably (50 to 320 nmol/L) likely reflecting differences in sample storage and assay conditions, timing of milk sample collection and the variability among the different population samples studied (O'Connor et al., 1991; O'Connor et al., 1997). Milk folate concentrations are known to increase with the duration of breastfeeding and from morning to night and they are lower in fore-versus hindmilk.
Because of the preferential partitioning of folate for milk synthesis, if maternal dietary folate intake is limiting during lactation, the concern is with the nutritional status and health of the mother herself and the impact of this on subsequent pregnancies. In addition to the amount of folate transferred into milk, women need extra folate during lactation to facilitate the synthesis of other constituents in milk (e.g. protein synthesis). While dietary folate requirements on a daily basis are less than during pregnancy, they are significantly greater than that of non-pregnant/non-lactating women. Adherence to the WHO guidance on duration and exclusivity of breastfeeding, would result in a greater net requirement for folate during lactation versus pregnancy. Among women who do not consume a folic acid supplement during either pregnancy or lactation, one can expect a further decline in blood folate concentrations immediately post-partum (Houghton et al., 2006; Mackey & Picciano, 1999; Qvist et al., 1986). Mackey and Picciano (1999) assessed the effects of dietary and supplemental folate intakes during lactation and found that unsupplemented women at 6 months had lower values for red blood cell folate and hemoglobin concentrations than supplemented mothers and that plasma homocysteine concentrations increased. Plasma homocysteine concentration is a nonspecific functional indicator of folate status of which an increase is indicative of compromised folate status. Again most of what we know about maternal folate status during lactation comes from studies that were conducted pre-fortification of the food supply. How fortification of the food supply in Canada has affected folate status during lactation is described below.
Where the food supply is not fortified with folic acid, several environmental factors have been associated with low blood folate concentrations. Nutritive factors include poor eating habits, low fruit and vegetable intake, stringent dieting for weight loss and food preparation practices which leech and breakdown endogenous folate content (eg. prolonged boiling or stewing of vegetables) (O'Connor, 1994; Picciano et al., 1994). Nonnutritive factors include drug and/or alcohol abuse, younger maternal age, past reproductive history (close birth spacing and number of pregnancies) and poverty. While a number of investigators report lower blood folate levels among smokers compared to non-smokers (Nakazawa et al., 1983; Ortega et al., 1994; Senti & Pilch, 1985; Witter et al., 1982); this relationship appears to be attenuated once researchers statistically control for dietary folate intake (Green et al., 1998b; Piyathilake et al., 1994). In other words, smokers may have poorer dietary folate intakes than non-smokers, and the reason they have lower blood folate concentrations may be due to their dietary folate intake and not smoking per se. Early studies also provided evidence that oral contraceptives may have negatively impact blood folate concentrations; however as the estrogen content of oral contraceptives has declined, so has the strength of the relationship (Green et al., 1998b; Sutterlin et al., 2003).
In addition to nutritive and non-nutritive factors that can influence blood folate concentrations, several SNPs have been identified which may not only influence blood folate concentration but the forms of folate available for metabolism. The most thoroughly studied of these SNPs, and the one with the greatest known impact on folate metabolism, is the 677C >T MTHFR variant discussed earlier in relation to Down Syndrome (Bailey, 2003; Ueland et al., 2001). This polymorphism involves a cytosine (C) to thymidine (T) substitution at nucleotide position 677, which is responsible for an alanine to valine substitution in the MTHFR enzyme (Frosst et al., 1995; Goyette et al., 1994). Intracellular folate homeostasis depends on MTHFR, a crucial enzyme in the folate pathway that catalyzes the irreversible conversion of 5,10-methyltetrahydrofolate to 5-methyltetrahydrofolate which results in the conversion of homocysteine to methionine (Jacques et al., 1996). Individuals with this mutation with marginal or poor intakes of dietary folate have higher plasma homocysteine and lower plasma folate concentrations than people with a wild type 677CC MTHFR gene (Blom, 1998). In North America, the MTHFR C677T polymorphism is a common mutation, with an allele frequency of approximately 35% (Friso et al., 2002; Frosst et al., 1995; Ma et al., 1997). Approximately 12-15% of Caucasian and Asians are homozygous (TT) for the mutation and up to 50% are heterozygous (CT) (Bailey, 2003; Ueland et al., 2001).
In many cases, the clinical sequelae described for the MTHFR C677T SNP are similar to that of folate deficiency. For instance, the presence of this SNP appears to be associated, at least in some studies, with an increased risk of cardiovascular disease, NTDs, adverse pregnancy outcomes, and Down Syndrome (Ueland et al., 2001). Further, the presence of the MTHFR C677T SNP appears to be related to the risk of cancers of the breast, endometrium, cervix, esophagus, stomach, and bladder (Kim, 2000; Ueland et al., 2001). Typically individuals that are homozygous for the MTHFR C677T variant, exhibit reduced folate status as measured by red blood cell folate concentration (Parle-McDermott et al., 2006). Regardless of folate status, the forms of folate in red blood cells do seem to be affected by this SNP (ie increase non-methylated forms with the variant), and it is speculated this may result in the redirection or preferential partitioning of folate through folate-dependant biochemical pathways (Davis et al., 2005; Smith et al., 2006; Smulders et al., 2007).
Choi et al. (2006) investigated the relationship between the MTHFR C677T SNP and the folate status of Korean women of childbearing age. These investigators found that neither the C677T nor an A1298C SNP in the MTHFR gene were strongly associated with decreased plasma folate concentration or elevated plasma homocysteine concentration. However, the A1298C polymorphism did significantly influence red blood cell folate concentration, more so than the C677T polymorphism. A study done by Kim et al. (2004) investigated the effect of the interaction between the MTHFR C677T SNP and serum B vitamin levels on serum homocysteine levels in pregnant Korean women (n=177). They reported that serum homocysteine levels in these women varied significantly with MTHFR genotype and serum folate levels. As others have concluded, these researchers suggest that better maternal folate and B12 status may lessen the effect that the MTHFR genotype has on serum homocysteine levels.
Periconceptional folic acid supplementation reduces the risk of NTDs by 50%-70% (Czeizel, 1993; Lumley et al., 2001; MRC Vitamin Study Research Group, 1991), yet many women do not take folic acid supplements before conception (Honein et al., 2001; Mathews et al., 1998). Despite many public-health campaigns in North America, a significant portion of women of reproductive age remain unaware of the importance of folic acid supplementation in prevention of NTDs and an even higher proportion, 65-65% do not follow recommendations despite this knowledge (Bailey et al., 2003; Lindsey et al., 2005). In fact, the March of Dimes in a Gallup Survey of American women (n=2,617), reported a reduction in daily folic acid supplement use between 2004 (40%) and 2005 (35%) (Lindsey et al., 2005). The proportion of American women of childbearing age that had knowledge the relationship between folic acid and birth defects remained unchanged at 25%. In a study conducted in the Canadian province of Quebec, 70% of pregnant women (n=1,240) reported they were aware of the role of folic acid in prevention of NTDs but only 25% had taken the recommended dose of folate during the periconceptional period (Morin et al., 2002). Groups of Canadians, particularly noncompliant with periconceptional folic acid supplement, are these of low educational status, younger women, immigrants and women with unplanned pregnancies.
Since folic acid awareness does not necessarily translate into behaviour change, and since neural tube closure occurs by the fourth week of pregnancy, a time when many women are unaware that they are pregnant, public health policy makers in Canada, in November 1998, mandated the fortification of white flour, enriched pasta and cornmeal with folic acid (Regulatory impact analysis statement, 1998). This intervention was expected to increase the average daily folic acid intake of women of childbearing age by 100 µg with almost nobody receiving more than 1 mg.
Ray et al. (2002a, 2002b) has extensively studied the effect of the Canadian folic acid fortification program on the folate status of adults. In a retrospective review of red blood cell concentrations (n=8,884) from a large laboratory database (MDS Laboratories), Ray et al. (2002b) reported that the mean red blood cell folate concentration rose from 680 (CI 669-692) nmol/L pre-fortification to 852 (841-862) nmol/L post-fortification of the food supply. The MDS laboratories, located in Toronto, Canada, provide diagnostic services to approximately one-third of community-based patients in the province of Ontario. The mean age of patients in this sample was 57.4 years and approximately 55% were women. The clinical reasons why the red blood folate concentrations were requested was not specified in the paper. In a second paper, Ray et al. (2002c) reported that the red blood cell folate concentrations of women (mean age 31.8 years [18-42 years]) from the same laboratory database pre- and post-fortification increased from 527 nmol/L to 741 nmol/L. Folate concentrations in both these reports were measured by a competitive protein binding assay with a maximum reporting limit of 1,450 nmol/L. In a population-based study from the province of Newfoundland in Canada, Liu et al. (2004) reported that the red blood cell folate concentrations of reproductive-aged women increased from 625 (CI 601-649) nmol/L pre-fortification to 818 (784-854) nmol/L post-fortification of the food supply. Folic acid fortification of foods has taken place in the US, Costa Rica and Chile and similar, or higher, increases in blood folate concentrations have been reported (Chen & Rivea, 2004; Choumenkovitch et al., 2001; Choumenkovitch et al., 2002; Hertrampf & Cortes, 2004; Hirsch et al., 2002; Jacques et al., 1999; Ray, 2004).
Canadian studies report a significant reduction in NTDs since folic acid fortification of the food supply. In the province of Quebec, De Wals et al. (2003) noted a 32% reduction in affected live births, elective terminations, and stillbirth certificates. Not all NTD cases may have been identified in this study due to the nonspecific manner in which deaths were classified. In the province of Ontario, Ray et al. (2002a) reported a 48% reduction in NTDs using antenatal screening records and a hospital discharge database to identify cases (Fig. 3). Gucciardi et al. (2002) examined the reduction of NTDs post folic acid fortification by enumerating cases reported in live birth, stillbirth, and hospital (but not clinic) therapeutic abortion records from the province of Ontario. This group found a 47% reduction in NTDs from 1995 to 1999. Lastly, Persad et al. (2002) investigated records of all livebirths, stillbirths and terminated pregnancies in the province of Nova Scotia from 1991 to 2000 and found a 54% decrease. Based on these studies, the fortification program appears to be preventing approximately 50% of NTDs which was the target goal for the policy. A study done by House et al. (2006) in the province of Newfoundland, where the incidence of NTDs have been historically high, showed a significant reduction in the number of NTDs from 4.67 (1992-1996) to 1.01 (1998-2002) per 1,000 total births.
As described above, prior to folic acid fortification of foods in North America, most pregnant and lactating women in Canada did not meet the recommended levels of folate intake from dietary sources alone. As such, health experts recommended that women take a folic acid-containing supplement while planning a pregnancy, and for the duration of pregnancy. No recommendations existed specific for lactation but many physicians further recommended that supplementation should be continued during lactation. Currently in Canada women are advised to consume a multi-vitamin containing 400 µg folic acid during the periconceptional period, for the duration of pregnancy and lactation (Health Canada, 2007). As available data suggest that many reproductive-age women continue to have red blood cell folate concentrations below 906 nmol/L, a value early in pregnancy shown to be an indicator of reduced risk of a NTD-affected pregnancy, advice by Health Canada for women to continue taking a folic acid-containing supplement during the periconceptional period seems prudent. Whether women continue to need a folic acid-containing supplement after closure of the neural tube, and during lactation since folic acid fortification of the food supply is less certain. Data from our own research group suggests that at mandated levels of folic acid fortification about one-third of well educated women still do not consume enough folate during pregnancy (post closure of the neural tube) and lactation from dietary sources alone (Sherwood et al., 2006). As actual levels of folic acid fortification of food in Canada may greatly exceed mandated levels (Quinlivan & Gregory, 2003b); the actual prevalence of inadequate intakes may be much lower. As surprising as it may seem, no one has directly measured the amount of folate added to the food supply in Canada. If we assume the amount of folic acid fortification is double mandated levels, as has been predicted by some in the United States, no women in our study would have had inadequate dietary intakes of folate (Sherwood et al., 2006). If this is, in fact true, a folic acid supplement is probably no longer necessary post-closure of the neural tube or during lactation unless a woman is capable of becoming pregnant. Therefore, one of the lessons learned from the Canadian folic acid experience is that it is crucial to determine and monitor the levels of folic acid that are used to enrich and fortify foods (Quinlivan & Gregory, 2003a).
Folates are present naturally in a variety of foods and occur in especially high levels in liver, dried peas and beans and green leafy vegetables including broccoli. Because of the volume consumed by North Americans, orange juice is often cited as the top contributor of naturally occurring folate in the diet (Sherwood et al., 2006). Sherwood reported post-folic acid fortification that the breads and grains food group surpassed the fruits and vegetable group in terms of the contribution of folate in the diet of pregnant and lactating women (Table 2). Breads and grains contributed 41.0% of total dietary folate to the diets of pregnant and lactating women in the Sherwood et al. (2006) study whereas fruits and vegetables (non-beverage sources) provided 21.2% of total dietary folate intake. Dietrich et al. (2005) also recently reported that after fortification, the category "bread, rolls, and crackers" became the single largest contributor of total folate in the U.S. diet, contributing 15.6% of total intake, surpassing vegetables, which were the number one folate food source prior to fortification.
Dietary habits differ considerably from country to country and, as such, the main sources of folate in a Korean diet differ greatly from those in a typical Canadian diet. As illustrated in Table 3 in which the dietary intakes of college students from a study by Han et al. (2005) are summarized, kimchi is the top contributor of dietary folate (21% for females). Kimchi is a traditional fermented cabbage dish that is consumed at nearly every meal. In addition to the quantity of kimchi consumed, it is noteworthy it is also very high in folate (115 ug/100 g) (Yon & Hyun, 2003). Rice was the next largest contributor of folate to the diet of Korean female college students (9%). Han et al. (2005) report that vegetables are usually prepared by boiling in Korea, and they predict that cooking may destroy approximately 29% of total dietary folate. It is difficult to predict the total intakes of dietary folate in Korea due to well described limitations in the food composition tables; however taking into account cooking losses, Han et al. (2005) estimated that the average folate intake of female college students was around 246 ug/d . This value is well below either the RDA (400 ug/d) or EAR (320 ug/d) for folate and is strikingly similar to the reported folate intakes of Canadian reproductive age females pre-fortification of the food supply (Green et al., 1998a; O'Connor, 1994; Picciano et al., 1994). Lim et al. (2000) measured the folate content in 24 hour food composites collected by reproductive age Korean women (n=91) and reported folate intakes of 145.8 ug/d.
In Korea, women consume a folic acid-containing supplement during pregnancy but as reported by Lee et al. (2005) most women (>80%) do not do so until after the 20th week of pregnancy, long after the neural tube has closed (Lim et al., 1999). Daly et al. (1995) report a maternal red blood cell folate above of 906 nmol/L during the periconceptional period is associated with a reduced risk of a NTD-affected pregnancy. Reported red blood cell folate concentrations of reproductive-age women in Korea fall well below this cut-off and are very similar to those reported for Canadian women pre-folic acid fortification of the food supply in Canada (Green et al., 1998a; Liu et al., 2004; Ray et al., 2002). For example, Lim et al. (2000), Han et al. (2005) and Choi et al. (2006) report the mean red blood cell folate concentrations of Korean women of childbearing age to be 513+142, 772+240 and 450 nmol/L, respectively. In the first trimester of pregnancy, Lee reported the red blood cell folate concentration of women not consuming a folic acid-containing supplement to be 613+152 nmol/L. Chang et al. (1993) studied 122 pregnant, lactating, and non-pregnant, non-lactating Korean women and found greater than 1/3rd had plasma folate concentrations falling below 6.8 nmol/L, a classic indicator of negative folate balance if maintained is a predictor of frank folate deficiency (eg. megaloblastic anemia).
Different methods of case ascertainment make comparison of the NTD rates between Korea and Canada impossible. There is some indication that the NTD rate in Korea may be lower than that in Canada pre-folic acid fortification of the food supply. Surveying representative members of the International Society for Pediatric Neurosurgery, Oi et al. (2003) reported a rate of spina bifida in Korea of 0.31 per 100,000 living Koreans compared to that of 1.27 per 100,000 Canadians. Using a Korean medical insurance database, Jung et al. (1999) reported the estimated prevalence of neural tube defects, including anencephaly, spina bifida and encephalocele, was 0.67-0.77 per 1,000 infants less than one year of age. In contrast, Ray et al. (2002) reported a reduction in the rate of open neural tube defects (anencephaly, spina bifida) from 1.13 to 0.58 per 1,000 pregnancies pre- to post-fortification in the province of Ontario, Canada. In the Canadian province with the highest rate of neural tube defects (Newfoundland), House et al. (2006) reported a reduction number of NTDs (anencephaly, spina bifida and encephalocele) from 4.67 to 1.01 per 1,000 total births. The latter two Canadian studies included pregnancies that were terminated early in their prediction of the NTD rate; our understanding of the Jung et al study is that they did not. Inclusion of terminated pregnancies in the NTD rates would increase the prevalence, but by how much is impossible to predict.
The purpose of this article was to describe the role of folate during female reproduction and specifically the periconceptional period, pregnancy (post-closure of the neural tube), and during lactation. The rationale for and history of recommending folic acid-containing supplements during the periconceptional period and pregnancy in Canada was described as was the folic acid fortification policy. Comparison of the published blood folate concentrations of reproductive-aged women in Korea suggests that their biochemical folate status is similar to those of Canadian women pre-fortification of the food supply. Certainly, the red blood cell folate concentrations of reproductive-age Korean women are well below that known to be maximally protective against neural tube defects. While a number of Korean authors acknowledge the significant limitations of currently available food composition tables to accurately estimate dietary folate intakes, estimated intakes appear to be inconsistent with those known to be protective against neural tube defects. The prevalence of neural tube defects may be lower in Korea than that in Canada pre-folic acid fortification of the food supply. Whether this reflects differences in genetic susceptibility for neural tube defects or merely differences in how cases of neural tube defects were attained remains to be determined. One of the significant lessons learned from the Canadian folic acid fortification experience over the last decade is the need to incorporate some form of on-going surveillance of actually how much folic acid is being added to the food supply. On-going surveillance is critical in evaluating the effectiveness and safety of the folic acid fortification strategy, and making adjustments to folate-containing supplements used in pregnancy and lactation.
Figures and Tables
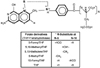 | Fig. 1
The chemical structure of folic acid or pterolylmonoglutamic acid. Other folate forms are denoted by the "R"-substitutions found in the box. |
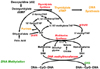 | Fig. 2
Simplified diagram of intracellular folate metabolism involving DNA biosynthesis and methylation. THF, tetrahydrofolate; MTHFR, methylenetetrahydrofolate; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; CpG, Cytosine-guanine dinucleotide sequence; CH3 , methyl group; DNMT(1, 3a, 3b), DNA methyltransferases; MTR, methionine synthase; MTRR, methionine synthase reductase; TS, Thymidylate synthase; DHFR, dihydrofolate reductase; dUMP, deoxyuridine monophosphate; dTMP, deoxythymidine monophosphate; SHMT, serine hydroxymethyltransferase; CBS, cystathionine β-synthase [diagram modified with permission (Kim, 2004a; Sohn et al., 2004)] |
 | Fig. 3
Quarterly prevalence of open neural tube defects(upper), spina bifida(middle), and anencephaly (lower) before and after (vertical dashed lines) folic acid food fortification. [reprinted with permission (Ray et al., 2002a)] |
 | Fig. 4
Rates of neural tube defects before and after fortification in regions with mandatory folic acid fortification. Numbers include livebirths, stillbirths, prenatally diagnosed cases and elective abortions (Chile with livebirths and stillbirths only ,USA with surveillance programmes with and without prenatal assessment) (reprinted with permission). [diagram modified with permission (Eichholzer et al., 2006)] |
Table 1
Estimated Average Requirements (EAR) and Recommended Dietary Allowance (RDA) for folate for adults, pregnant and lactating women (Institue of Medicine, 1998)

Table 2
Major contributors of folate (µg, uncorrected for bioavailability) in the diets of a sample of Canadian pregnant (n=61) and lactating women (n=60)
(Sherwood et al., 2006)

Table 3
Major contributors of folate (µg, uncorrected for bioavailability) in the diets of healthy Korean college females age 18-27 years (n=62) (Han et al., 2005)

References
1. American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2005. 115:496–506.
2. Badovinac RL, Werler MM, Williams PL, Kelsey KT, Hayes C. Folic acid-containing supplement consumption during pregnancy and risk for oral clefts: a meta-analysis. Birth Defects Res A Clin Mol Teratol. 2007. 79:8–15.

3. Bailey LB. Folate, methyl-related nutrients, alcohol, and the MTHFR 677CT→ polymorphism affect cancer risk: intake recommendations. J Nutr. 2003. 133:3748S–3753S.

4. Bailey LB, Rampersaud GC, Kauwell GP. Folic acid supplements and fortification affect the risk for neural tube defects,vascular disease and cancer; evolving science. J Nutr. 2003. 133:1961S–1968S.
5. Baumslag N, Edelstein T, Metz J. Reduction of incidence of prematurity by folic acid supplementation in pregnancy. Br Med J. 1970. 1:16–17.

6. Blackburn S, Loper DL. Blackburn S, editor. The hematologic and hemostatic systems. Maternal, fetal, and neonatal physiology: a clinical perspective. 1992. Philadelphia. USA: Saunders Company;159–200.
7. Blom HJ. Mutated 5,10-methylenetetrahydrofolate reductaseand moderate hyperhomocysteinaemia. Eur J Pediatr. 1998. 157:S131–S134.
8. Blom HJ, Shaw GM, den Heijer M, Finnell RH. Neural tube defects and folate: case far from closed. Nat Rev Neurosci. 2006. 7:724–731.

9. Botto LD, Khoury MJ, Mulinare J, Erickson JD. Periconceptional multivitamin use and the occurrence of conotruncal heart defects: results from a population-based, case-control study. Pediatrics. 1996. 98:911–917.

10. Chanarin I. The megaloblastic anaemias. 1969. London. UK: Blackwell.
11. Chang NS, Kang MH, Paik HY, Kim IH, Cho YW, Park SC, Shin YW. Serum folate and iron levels of pregnant, lactating adn non-pregnant, non-lactating women. The Korean Journal of Nutrition. 1993. 26:67–75.
12. Chen LT, Rivera MA. The Costa Rican experience: reduction of neural tube defects following food fortification programs. Nutr Rev. 2004. 62:S40–S43.

13. Choi JH, Kim HA, Lim HS. MTHFR polymorphism and folate status of Korean women of childbearing age. Nutritional Sciences. 2006. 9:35–41.
14. Choumenkovitch SF, Jacques PF, Nadeau MR, WIlson PW, Rosenberg IH, Selhub J. Folic acid fortification increases red blood cell foalte concentrations in the Framingham study. J Nutr. 2001. 131:3277–3280.

15. Choumenkovitch SF, Selhub J, WIlson PW, Rader JI, Rosenberg IH, Jacques PF. Folic acid intake from fortification in United States exceeds predictions. J Nutr. 2002. 132:2792–2798.

16. Czeizel AE. Prevention of congenital abnormalities bypericonceptional multivitamin supplementation. BMJ. 1993. 306:1645–1648.

17. Czeizel AE. Reduction of urinary tract and cardiovascular defects by periconceptional multivitamin supplementation. Am J Med Genet. 1996. 62:179–183.

18. Czeizel AE, Tímár L, Sárközi A. Dose-dependent effect of folic acid on the prevention of orofacial clefts. Pediatrics. 1999. 104:e66.

19. Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels and neural tube defects. Implications for prevention. JAMA. 1995. 274:1698–1702.

20. Davis SR, Quinlivan EP, Shelnutt KP, Maneval DR, Ghandour H, Capdevila A, Coats BS, Wagner C, Selhub J, Bailey LB, Shuster JJ, Stacpoole PW, Gregory JF 3rd. The methylenetetrahydrofolate reductase 677CT→ polymorphism and dietary folate restriction affect plasma one-carbon metabolites and red blood cell folate concentrations and distribution in women. J Nutr. 2005. 135:1040–1044.

21. De Wals P, Rusen ID, Lee NS, Morin P, Niyonsenga T. Trend in prevalence of neural tube defects in Quebec. Birth Defects Res A Clin Mol Teratol. 2003. 67:919–923.

22. Dietrich M, Brown CJ, Block G. The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States. J Am Coll Nutr. 2005. 24:266–274.

23. Eichholzer M, Tonz O, Zimmermann R. Folic acid: a public-health challenge. Lancet. 2006. 367:1352–1361.

24. Eskes TK. Abnormal folate metabolism in mothers with Down syndrome offspring: review of the literature. Eur J Obstet Gynecol Reprod Biol. 2006. 124:130–133.

25. French AE, Grant R, Weitzman S, Ray JG, Vermeulen MJ, Sung L, Greenberg M, Koren G. Folic acid food fortification is associated with a decline in neuroblatoma. Clin Pharmacol Ther. 2003. 74:288–294.

26. Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, Olivieri O, Jacques PF, Rosenberg IH, Corrocher R, Selhub J. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA. 2002. 99:5606–5611.

27. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995. 10:111–113.

28. Goyette P, Sumner JS, Milos R, Duncan AM, Rosenblatt DS, Matthews RG, Rozen R. Human methylenetetrahydrofolate reductase: isolation of cDNA, mapping and mutation identification. Nat Genet. 1994. 7:195–200.

29. Green TJ, Allen OB, O'Connor DL. A three-day weighed food record and a semiquantitative food-frequency questionnaire are valid measures for assessing the folate and vitamin B-12 intakes of women aged 16 to 19 years. J Nutr. 1998a. 128:1665–1671.

30. Green TJ, Houghton LA, Donovan U, Gibson RS, O'Connor DL. Oral contraceptives did not affect biochemical folate indexes and homocysteine concentrations in adolescent females. J Am Diet Assoc. 1998b. 98:49–55.

31. Gucciardi E, Pietrusiak MA, Reynolds DL, Rouleau J. Incidence of neural tube defects in Ontario, 1986-1999. CMAJ. 2002. 167:237–240.
32. Han YH, Yon M, Hyun TH. Folate intake estimated with an updated database and its association to blood folate and homocysteine in Korean college students. Eur J Clin Nutr. 2005. 59:246–254.

33. Exclusive breastfeeding duration. Health Canada. 2004. Accessed on 2004. http://www.hc-sc.gc.ca/fn-an/nutrition/child-enfant/infant-nourisson/excl_bf_dur-dur_am_excl_e.html.
34. Eating Well with Canada's Food Guide: A Resource for Educators and Communicators. Health Canada. 2007. Accessed on 2007. http://www.healthcanada.gc.ca/foodguide.
35. Hertrampf E, Cortes F. Folic acid fortification of wheat flour: Chile. Nutr Rev. 2004. 62:S44–S48. discussion S49.

36. Hirsch S, de la Maza P, Barrera G, Gattas V, Petermann M, Bunout D. The Chilean flour folic acid fortification program reduces serum homocysteine levels and masks vitamin B-12 deficiency in elderly people. J Nutr. 2002. 132:289–291.

37. Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001. 285:2981–2986.

38. Houghton LA, Sherwood KL, Pawlosky R, Ito S, O'Connor DL. [6S]-5-Methyltetrahydrofolate is at least as effective as folic acid in preventing a decline in blood folate concentrations during lactation. Am J Clin Nutr. 2006. 83:842–850.

39. House JD, March SB, Ratnam MS, Crowley M, Friel JK. Improvements in the status of folate and cobalamin in pregnant Newfoundland women are consistent with observed reductions in the incidence of neural tube defects. Can J Public Health. 2006. 97:132–135.

40. Institute of Medicine. Food and Nutrition Board. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. 1998. Washington, DC. USA: National Academy Press.
41. Iyengar L, Rajalakshmi K. Effect of folic acid supplement on birth weights of infants. Am J Obstet Gynecol. 1975. 122:332–336.

42. Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, Selhub J, Rozen R. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996. 93:7–9.

43. Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate andtotal homocysteine concentrations. New Engl J Med. 1999. 340:1449–1454.

44. Jugessur A, Wilcox AJ, Lie RT, Murray JC, Taylor JA, Ulvik A, Drevon CA, Vindenes HA, Abyholm FE. Exploring the effects of methylenetetrahydrofolate reductase gene variants C677T and A1298C on the risk of orofacial clefts in 261 Norwegian case-parent triads. Am J Epidemiol. 2003. 157:1083–1091.

45. Jung SC, Kim SS, Yoon KS, Lee JS. Prevalence of congenital malformations and genetic diseases in Korea. J Hum Genet. 1999. 44:30–34.

46. Kim KN, Kim YJ, Chang N. Effects of the interaction between the C677T 5,10-methylenetetrahydrofolate reductase polymorphism and serum B vitamins on homocysteine levels in pregnant women. Eur J Clin Nutr. 2004. 58:10–16.

47. Kim YI. Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem. 1999. 10:66–88.

48. Kim YI. Methylenetetrahydrofolate reductase polymorphisms, folate, and cancer risk: a paradigm of gene-nutrient interactions in carcinogenesis. Nutr Rev. 2000. 95:205–209.

49. Kim YI. Role of folate in colon cnacer development and progression. J Nutr. 2003. 133:3731–3795.
50. Kim YI. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev. 2004a. 13:511–519.
51. Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004b. 80:1123–1128.

52. Kleinman RE. Pediatric Nutrition Handbook. Breastfeeding. 2004. 3:Fifth Edition. USA: American Academy of Pediatrics;55–86.
53. Lee JI, Lee JA, Lim HS. Effect of time of initiation and dose of prenatal iron and folic acid supplementation on iron and folate nutriture of Korean women during pregnancy. Am J Clin Nutr. 2005. 82:843–849.

54. Li DK, Daling JR, Mueller BA, Hickok DE, Fantel AG, Weiss NS. Periconceptional multivitamin use in relation to the risk of congenital urinary tract anomalies. Epidemiology. 1995. 6:212–218.

55. Lim HS, Jin HO, Lee JA. Dietary intakes and status of folate in Korean women of child-bearing potential. The Korean Journal of Nutrition. 2000. 33:296–303.
56. Lim HS, Lee JI, Lee JA. Folate status of Korean pregnant women and their pregnancy outcomes - a cross-section study. The Korean Journal of Nutrition. 1999. 32:592–597.
57. Lindsey LLM, Carter H, Pruc C, Mulinare J. Use of dietary supplements containing folic acid among women of childbearing age-United States 2005. MMWR Weekly. 2005. 54:955–958.
58. Liu S, West R, Randell E, Longerich L, O'Connor KS, Scott H, Crowley M, Lam A, Prabhakaran V, McCourt C. A comprehensive evaluation of food fortification with folic acid for the primary prevention of neural tube defects. BMC Pregnancy Childbirth. 2004. 4:20.

59. Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000. 71:121–138.

60. Lumley J, Watson L, Watson M, Bower C. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001. CD001056.

61. Ma J, Stampfer MJ, Giovannucci E, Artigas C, Hunter DJ, Fuchs C, Willett WC, Selhub J, Hennekens CH, Rozen R. Methylenetetrahydrofolate reductase polymorphism, dietary interactions and risk of colorectal cancer. Cancer Res. 1997. 57:1098–1102.
62. Mackey AD, Picciano MF. Maternal folate status during extended lactation and the effect of supplemental folic acid. Am J Clin Nutr. 1999. 69:285–292.

63. Mathews F, Yudkin P, Neil A. Folates in the periconceptional period: are women getting enough? Br J Obstet Gynaecol. 1998. 105:954–959.

64. Mills JL, Kirke PN, Molloy AM, Burke H, Conley MR, Lee YJ, Mayne PD, Weir DG, Scott JM. Methylenetetrahydrofolate reductase thermolabile variant and oral clefts. Am J Med Genet. 1999. 86:71–74.

65. Morin P, De Wals P, Noiseux M, Niyonsenga T, St-Cyr-Tribble D, Tremblay C. Pregnancy planning and folic acid supplement use: results from a survey in Quebec. Prev Med. 2002. 35:143–149.

66. MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991. 338:131–137.
67. Nakazawa Y, Chiba K, Imatoh N, Kotorii T, Sakamoto T. Ishizaki T: Serum folic acid levels and antipyrine clearance rates in smokers and non-smokers. Drug Alcohol Depend. 1983. 11:201–207.

68. O'Connor DL. Koren G, editor. The folate status of Canadian women. Folic acid for the prevention of neural tube defects. 1994. Toronto. Canada: The Motherisk Program.
69. O'Connor DL, Green T, Picciano MF. Maternal folate status and lactation. J Mammary Gland Biol Neoplasia. 1997. 2:279–289.
70. O'Connor DL, Tamura T, Picciano MF. Pteroylpolyglutamates in human milk. Am J Clin Nutr. 1991. 53:930–934.
71. Oi S. Current status of prenatal management of fetal spina bifida in the world: worldwide cooperative survey on the medicoethical issue. Childs Nerv Syst. 2003. 19:596–599.

72. Ortega RM, Lopez-Sobaler AM, Gonzalez-Gross MM, Redondo RM, Marzana I, Zamora MJ, Andres P. Influence of smoking on folate intake and blood folate concentrations in a group of elderly Spanish men. J Am Coll Nutr. 1994. 13:68–72.

73. Parle-McDermott A, Mills JL, Molloy AM, Carroll N, Kirke PN, Cox C, Conley MR, Pangilinan FJ, Brody LC, Scott JM. The MTHFR 1298CC and 677TT genotypes have opposite associations with red cell folate levels. Mol Genet Metab. 2006. 88:290–294.

74. Persad VL, Van den Hof MC, Dube JM, Zimmer P. Incidence of open neural tube defects in Nova Scotia after folic acid fortification. CMAJ. 2002. 167:241–245.
75. Pfeiffer CM, Rogers LM, Bailey LB, Gregory JF 3rd. Absorption of folate from fortified cereal-grain products and of supplemental folate consumed with or without food determined byusing a dual-label stable-isotope protocol. Am J Clin Nutr. 1997. 66:1388–1397.

76. Picciano M, Green T, O'Connor D. The folate status of women and health. Nutr Today. 1994. 29:20–29.

77. Piyathilake CJ, Macaluso M, Hine RJ, Richards EW, Krumdieck CL. Local and systemic effects of cigarette smoking on folate and vitamin B-12. Am J Clin Nutr. 1994. 60:559–566.

78. Quinlivan EP, Gregory JF 3rd. Effect of food fortification on folic acid intake in the United States. Am J Clin Nutr. 2003a. 77:221–225.

79. Quinlivan EP, Gregory JF 3rd. The impact of food fortification on folic acid intake in Canada. Can J Public Health. 2003b. 94:154.
80. Qvist I, Abdulla M, Jagerstad M, Svensson S. Iron, zinc and folate status during pregnancy and two months after delivery. Acta Obstet Gynecol Scand. 1986. 65:15–22.

81. Ray JG. Meta-analysis of hyperhomocysteinemia as a risk factor for venous thromboembolic disease. Arch Intern Med. 1998. 158:2101–2106.

83. Ray JG, Meier C, Vermeulen MJ, Boss S, Wyatt PR, Cole DE. Association of neural tube defects and folic acid food fortification in Canada. Lancet. 2002a. 360:2047–2048.

84. Ray JG, Meier C, Vermeulen MJ, Wyatt PR, Cole DE. Association between folic acid food fortification and congenital orofacial clefts. J Pediatr. 2003. 143:805–807.

85. Ray JG, Vermeulen MJ, Boss SC, Cole DE. Declining rate of folate insufficiency among adults following increased folic acid food fortification in Canada. Can J Public Health. 2002b. 93:249–253.

86. Ray JG, Vermeulen MJ, Boss SC, Cole DE. Increased red cell folate concentrations in women of reproductive age after Canadian folic acid food fortification. Epidemiology. 2002c. 13:238–240.

87. Regulatory impact analysis statement. SOR/98-550, Canada Gazette Part II. 1998. 3029–3033.
88. Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Dietary and serum folate: their influence on the outcome of pregnancy. Am J Clin Nutr. 1996. 63:520–525.

89. Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002. 346:476–483.

90. Senti FR, Pilch SM. Analysis of folate data from the second National Health and Nutrition Examination Survey (NHANES II). J Nutr. 1985. 115:1398–1402.

91. Shaw GM, O'Malley CD, Wasserman CR, Tolarova MM, Lammer EJ. Maternal periconceptional use of multivitamins and reduced risk for conotruncal heart defects and limb deficiencies among offspring. Am J Med Genet. 1995. 59:536–545.

92. Shaw GM, Rozen R, Finnell RH, Todoroff K, Lammer EJ. Infant C677T mutation in MTHFR, maternal periconceptional vitamin use, and cleft lip. Am J Med Genet. 1998. 80:196–198.

93. Sherwood KL, Houghton LA, Tarasuk V, O'Connor DL. One-third of pregnant and lactating women may not be meeting their folate requirements from diet alone based on mandated levels of folic acid fortification. J Nutr. 2006. 136:2820–2826.

94. Smith DE, Kok RM, Teerlink T, Jakobs C, Smulders YM. Quantitative determination of erythrocyte folate vitamer distribution by liquid chromatography-tandem mass spectrometry. Clin Chem Lab Med. 2006. 44:450–459.

95. Smithells RW, Sheppard S, Schorah CJ. Vitamin deficiencies and neural tube defects. Arch Dis Child. 1976. 51:944–950.
96. Smulders YM, Smith DE, Kok RM, Teerlink T, Gellekink H, Vaes WH, Stehouwer CD, Jakobs C. Red blood cell folate vitamer distribution in healthy subjects is determined by the methylenetetrahydrofolate reductase C677T polymorphism and by the total folate status. J Nutr Biochemr. 2007. 04. 04. [Epub ahead of print].

97. Sohn KJ, Croxford R, Yates Z, Lucock M, Kim YI. Effect of the methylenetetrahydrofolate reductase C677T polymorphism on chemosensitivity of colon and breast cancer cells to 5-fluorouracil and methotrexate. J Natl Cancer Inst. 2004. 96:134–144.

98. Sutterlin MW, Bussen SS, Rieger L, Dietl J, Steck T. Serum folate and Vitamin B12 levels in women using modern oral contraceptives (OC) containing 20 microg ethinyl estradiol. Eur J Obstet Gynecol Reprod Biol. 2003. 107:57–61.
100. Tolarova M. Periconceptional supplementation with vitamins and folic acid to prevent recurrence of cleft lip. Lancet. 1982. 2:217.

101. Ueland PM, Hustad S, Schneede J, Refsum H, Vollset SE. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci. 2001. 22:195–201.

102. van Rooij IA, Vermeij-Keers C, Kluijtmans LA, Ocke MC, Zielhuis GA, Goorhuis-Brouwer SM, van der Biezen JJ, Kuijpers-Jagtman AM, Steegers-Theunissen RP. Does the interaction between maternal folate intake and the methylenetetrahydrofolate reductase polymorphisms affect the risk of cleft lip with or without cleft palate? Am J Epidemiol. 2003. 157:583–591.

103. Vollset SE, Refsum H, Irgens LM, Emblem BM, Tverdal A, Gjessing HK, Monsen AL, Ueland PM. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. Am J Clin Nutr. 2000. 71:962–968.

104. Wilcox AJ. On the importance-and the unimportance-of birthweight. Int J Epidemiol. 2001. 30:1233–1241.
105. Wilcox AJ, Lie RT, Solvoll K, Taylor J, McConnaughey DR, Abyholm F, Vindenes H, Vollset SE, Drevon CA. Folic acid supplements and risk of facial clefts: national population based case-control study. BMJ. 2007. 334:464.

106. Witter FR, Blake DA, Baumgardner R, Mellits ED, Niebyl JR. Folate, carotene, and smoking. Am J Obstet Gynecol. 1982. 144:857.

107. Global Strategies for Infant and Young Child Feeding. Resolution Passes at: Fifty-fourth World Health Assembly; May 9. World Health Organization. 2001. Accessed on 2001. http://www.who.int/child-adolescent-health/NUTRITION/global_strategy.htm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download