Abstract
The anti-obesity effects of fermented black bean were tested with mice fed a high fat diet for seven weeks. Body weight gain and feed efficiency ratio (FER) in the high fat diet control (HC) group were markedly higher, compared with those of the normal control (NC) group, but were significantly lower in the 2% black bean powder supplemented high fat diet (BB) group and 2% black bean powder fermented by M. pilosus supplemented high fat diet (BBM) group, compared with those of the HC group. Food intake in the HC and BB groups was significantly lower than that of the NC and BBM groups. Water intake in the HC group was significantly lower than that of the NC group, but was higher in the BB and BBM groups, compared with that of the HC group. On the other hand, relative liver and kidney weight in the HC group was lower than that of the NC group, but was higher in the BB and BBM groups, compared with that of the HC group. In addition, whereas epididymal fat weight in the HC group was markedly higher than that of the NC group, it was significantly lower in the BB and BBM groups, compared with that of the HC group. Meanwhile, hepatic GSH in the HC group was significantly lower than that of the NC group, but was slightly higher in the BB and BBM groups, compared with that of the HC group. Although hepatic LPO in the HC group was dramatically higher than that of the NC group, it was significantly lower in the BB and BBM groups, compared with that of the HC group. In addition, serum TG, total cholesterol, and LDL-cholesterol in the HC group was significantly higher than that of the NC group, but was significantly lower in the BB and BBM groups, compared with that of the HC group. On the contrary, HDL-cholesterol in the HC group was significantly lower than that of the NC group, but was higher in the BB and BBM groups, compared with that of the HC group. In addition, activity of XOR D type in the HC group was lower than that of the NC group, but was slightly higher in the BB and BBM groups, compared with that of the NC group. Activities of ROS scavenging enzymes, such as SOD, GPX, and GST in the HC group were significantly lower than those of the NC group, but were significantly higher in the BB and BBM groups, compared with those of the HC group. In addition, serum ALT activity in the HC and BB groups was higher than that of the NC group, but was significantly lower in the BB and BBM groups, compared with that of the HC group. In histopathological findings, hepatic fat accumulation in the HC group was higher than that of the NC group, but was lower in the BBM group, compared with that of the HC and BB groups. In particular, antiobese, hypolipidemic, and antifatty liver effect of black bean powder fermented by M. pilosus was specifically higher than that of non-fermented steamed black bean. In conclusion, the constituents of black bean fermented by Monascus pilosus have been proven to not only inhibit obesity and hyperlipidemia but also decrease hepatic fat accumulation in high fat diet-induced obese mice.
Figures and Tables
 | Fig. 1Effects of black bean fermented by M. pilosus on the content of glutathione (GSH) and lipid peroxide (LPO) in the liver of mouse fed high fat diets for 7 weeks. Values are Mean ± standard deviation (n = 6), different superscripts on the bars indicate significant difference (p < 0.05). Units of GSH and LPO are represented as µmole/g tissue, respectively. |
 | Fig. 2Effects of black bean fermented by M. pilosus on the content of triglyceride (TG), total cholesterol, HDL-cholesterol and LDL-cholesterol of in serum of the mouse fed high fat diets for 7 weeks. Values are Mean ± standard deviation (n = 6), different superscripts on the bars indicate significant difference (p < 0.05). |
 | Fig. 3Effects of black bean fermented by M. pilosus on the activities of hepatic XOR D and O type enzyme in the mouse fed high fat diets for 7 weeks. Values are Mean ± standard deviation (n = 6), different superscripts on the bars indicate significant difference (p < 0.05). Units of XOR D type and O type activity are represented as uric acid nmole/min/mg protein, respectively. |
 | Fig. 4Effects of black bean fermented by M. pilosus on the activities of hepatic SOD, GST, GPX and serum ALT in the mouse fed with high fat diets for 7 weeks. Values are Mean ± standard deviation (n = 6), different superscripts on the bars indicate significant difference (p < 0.05). Units of SOD, GST, GPX and ALT are represented as U/mg protein, thioether nmole/min/mg protein, NADPH nmole/min/mg protein and Karmen units, respectively. |
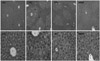 | Fig. 5Light microscopic photographs of liver tissue (bar: 30 µm) of mouse fed black bean fermented by M. pilosus supplemented high fat diets for 7 weeks (HE stain). See Table 1. C; central vein. Arrows (H1 and, H2) in HC: focal infiltration of inflammatory cells. |
Table 1
Experimental groups and ingredients of diets (%)
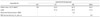
1) NC: normal control group, HC: high fat diet control group, BB: 2% steamed black bean powder supplemented high fat diet group, BBM: 2% black bean powder fermented by M. pilosus supplemented high fat diet group 2) The diets for animal experiments manufactured in the PMI Nutrition, LLC, Brentwood, MO, USA. Guaranteed analysis: crude protein 18%, crude fat 5%, crude fiber 5%, ash 8%
Table 2
Effects of black bean fermented by M. pilosus on body weight gain and feed efficiency ratio of mouse fed high fat supplemented diets for 7 weeks

References
2. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114(12):1752–1761.

3. Gwinner W, Scheuer H, Haller H, Brandes RP, Groene HJ. Pivotal role of xanthine oxidase in the initiation of tubulointerstitial renal injury in rats with hyperlipidemia. Kidney Int. 2006; 69(3):481–487.

4. Oben JE, Enyegue DM, Fomekong GI, Soukontoua YB, Agbor GA. The effect of Cissus quadrangularis (CQR-300) and a Cissus formulation (CORE) on obesity and obesity-induced oxidative stress. Lipids Health Dis. 2007; 6:4.
5. Juzlova P, Martinkova L, Kren V. Secondary metabolites of the fungus Monascus: a review. J Ind Microbiol. 1996; 16(3):163–170.
6. Kang MR, Kim JY, Hyun YJ, Kim HJ, Yeo HY, Song YD, Lee JH. The effect of red-yeast-rice supplement on serum lipid profile and glucose control in subjects with impaired fasting glucose or impaired glucose tolerance. Korean J Nutr. 2008; 41(1):31–40.
7. Inoue K, Mukaiyama Y, Tsuji K, Tanabe N, Tarui S, Abe S, Takahashi M. Effect of beni-koji extracts on blood pressure in primary hypertensive volunteers. Jpn J Nutr. 1995; 53(4):263–271.

8. Yasukawa K, Takahashi M, Yamanouchi S, Takido M. Inhibitory effect of oral administration of Monascus pigment on tumor promotion in two-stage carcinogenesis in mouse skin. Oncology. 1996; 53(3):247–249.

9. Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H. Blood-pressure-lowering effect of a novel fermented milk containing γ-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr. 2003; 57(3):490–495.

10. Lee SI, Kim JW, Lee YK, Yang SH, Lee IA, Suh JW, Kim SD. Anti-obesity effect of Monascus pilosus mycelial extract in high fat diet induced obese rats. J Korean Soc Appl Biol Chem. 2011; 54(3):197–205.

11. Lee SI, Kim JW, Lee YK, Yang SH, Lee IA, Suh JW, Kim SD. Protective effect of Monascus pilosus mycelial extract on hepatic damage in high-fat diet induced-obese rats. J Korean Soc Appl Biol Chem. 2011; 54(3):206–213.

12. Kim CS, Rhee SH, Kim I. Studies on production and characteristics of edible red color pigment produced by mold (Monascus sp.). Korean J Food Sci Technol. 1977; 9(4):277–283.
13. Broder CU, Koehler PE. Pigments produced by Monascus purpureus with regard to quality and quantity. J Food Sci. 1980; 45(3):567–569.

14. Tsukioka M, Hiroi T, Suzuki T, Konno T. Pigment production by mutants of Monascus anka (Studies on alcoholic beverage production using genus Monascus. Part I. Nippon Nogeikagaku Kaishi. 1986; 60(6):451–455.
15. Blanc PJ, Loret MO, Goma G. Production of citrinin by various species of Monascus. Biotechnol Lett. 1995; 17(3):291–294.

16. Liao HF, Chen YJ, Yang YC. A novel polysaccharide of black soybean promotes myelopoiesis and reconstitutes bone marrow after 5-flurouracil- and irradiation-induced myelosuppression. Life Sci. 2005; 77(4):400–413.

17. Shin HC, Sung HS, Lee YS, Sohn HS. Nutritional adequacy and beneficial effects of soy formula. Korea Soyb Dig. 2001; 18(2):10–32.
18. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18(6):499–502.

19. Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957; 28(1):56–63.

21. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95(2):351–358.

22. Stirpe F, Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem. 1969; 244(14):3855–3863.
23. Martin JP Jr, Dailey M, Sugarman E. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys. 1987; 255(2):329–336.

24. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974; 249(22):7130–7139.
25. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70(1):158–169.
26. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1):265–275.

27. Kim JY, Nolte LA, Hansen PA, Han DH, Ferguson K, Thompson PA, Holloszy JO. High-fat diet-induced muscle insulin resistance: relationship to visceral fat mass. Am J Physiol Regul Integr Comp Physiol. 2000; 279(6):R2057–R2065.

28. Lee JM, Cho WK, Park HJ. Effects of chitosan treated with enzymatic methods on glucose and lipid metabolism in rats. Korean J Nutr. 1998; 31(7):1112–1120.
29. Yu MH, Lee HJ, Im HG, Hwang Bo MH, Kim HJ, Lee IS. The effects of Kimchi with Monascus purpureus on the body weight gain and lipid metabolism in rats fed high fat diet. J Life Sci. 2005; 15(4):536–541.
30. Wu YG, Xia LL, Lin H, Zhou D, Qian H, Lin ST. Prevention of early liver injury by breviscapine in streptozotocin-induced diabetic rats. Planta Med. 2007; 73(5):433–438.

31. Gregoire FM, Zhang Q, Smith SJ, Tong C, Ross D, Lopez H, West DB. Diet-induced obesity and hepatic gene expression alterations in C57BL/6J and ICAM-1-deficient mice. Am J Physiol Endocrinol Metab. 2002; 282(3):E703–E713.
32. Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, Kaneko S. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008; 57(8):1071–1077.

33. Ha SK, Chae C. Inducible nitric oxide distribution in the fatty liver of a mouse with high fat diet-induced obesity. Exp Anim. 2010; 59(5):595–604.

34. Wang RS, Nakajima T, Honma T. Different change patterns of the isozymes of cytochrome P450 and glutathione S-transferases in chemically induced liver damage in rat. Ind Health. 1999; 37(4):440–448.

35. Kang MH, Lee JH, Lee JS, Kim JH, Chung HK. Effects of acorn supplementation on lipid profiles and antioxidant enzyme activities in high fat diet-induced obese rats. Korean J Nutr. 2004; 37(3):169–175.
36. Eybl V, Kotyzová D, Bludovská M. The effect of curcumin on cadmium-induced oxidative damage and trace elements level in the liver of rats and mice. Toxicol Lett. 2004; 151(1):79–85.

37. Lee IS, Lee S, Lee IZ. Effects of tissue cultured ginseng on blood glucose and lipids in streptozotocin-induced diabetic rats. Korean J Food Sci Technol. 2003; 35(2):280–285.
38. Park BH, Beck KY, Lee SI, Kim SD. Effect of chitosan-ascorbate containing soyfiber beni-koji on body weight and lipid content of obesity rats aid induced from high fat diet. J East Asian Soc Diet Life. 2006; 16(6):663–669.
39. Li C, Zhu Y, Wang Y, Zhu JS, Chang J, Kritchevsky D. Monascus purpureus-fermented rice (red yeast rice): a natural food product that lowers blood cholesterol in animal models of hypercholesterolemia. Nutr Res. 1988; 18(1):71–81.

40. Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VL. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr. 1999; 69(2):231–236.

41. Wang J, Lu Z, Chi J, Wang W, Su M, Kou W, Yu P, Yu L, Chen L, Zhu JS, Chang J. Multicenter clinical trial of the serum lipid-lowering effects of a Monascus purpureus (Red Yeast) rice preparation from traditional Chinese medicine. Curr Ther Res Clin Exp. 1997; 58(12):964–978.

42. Arad Y, Ramakrishnan R, Ginsberg HN. Lovastatin therapy reduces low density lipoprotein apoB levels in subjects with combined hyperlipidemia by reducing the production of apoB-containing lipoproteins: implications for the pathophysiology of apoB production. J Lipid Res. 1990; 31(4):567–582.

43. Park GY, Lee SJ, Im JG. Effects of green tea catechin on cytochrome p450, xanthine oxidase activities in liver and liver damage in streptozotocin induced diabetic rats. J Korean Soc Food Sci Nutr. 1997; 26(5):901–907.
44. Ham YK, Kim SW. Protective effects of plant extracts on the hepatocytes of rat treated with carbon tetrachloride. J Korean Soc Food Sci Nutr. 2004; 33(8):1246–1251.

45. Hashim MS, Lincy S, Remya V, Teena M, Anila L. Effect of polyphenolic compounds from Coriandrum sativum on H2O2-induced oxidative stress in human lymphocytes. Food Chem. 2005; 92(4):653–660.

46. Flohe L, Günzler WA, Schock HH. Glutathione peroxidase: a selenoenzyme. FEBS Lett. 1973; 32(1):132–134.

47. Lee J, Jeong JY, Cho YS, Park SK, Kim K, Kim MJ, Lee MK. Effect of young Phragmites communis leaves powder on lipid metabolism and erythrocyte antioxidant enzyme activities in high-fat diet fed mice. J Korean Soc Food Sci Nutr. 2010; 39(5):677–683.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download