Abstract
This study was conducted in order to investigate the association between hypertension and oxidative stress-related parameters and to evaluate these parameters in subclinical hypertensive patients and normotensive subjects living in Korea. We attempted to determine whether oxidative stress-related parameters would differ between two groups of 227 newly-diagnosed, untreated (systolic blood pressure (BP) ≥ 130 mmHg and diastolic BP ≥ 85 mmHg) and 130 normotensive subjects (systolic BP < 120 mmHg and diastolic BP < 80 mmHg). General characteristics of the subjects were collected using a simple questionnaire. From subjects' blood, degree of DNA damage in lymphocytes, the activities of erythrocyte superoxide dismutase, catalase, and glutathione peroxidase, level of plasma total radical-trapping antioxidant potential (TRAP), glutathione, and anti-oxidative vitamins, as well as plasma lipid profiles and conjugated diene (CD) were analyzed. Evaluation of the associations of oxidative stress-related parameters with blood pressure of the subjects was performed using Pearson partial correlation and multivariate logistic regression analysis after adjusting for confounding factors. Several oxidative stress-related parameters were higher in subclinical hypertensive patients than in normotensive subjects. Plasma levels of α-tocopherol, β-carotene, TRAP, and activity of GSH-px were significantly lower in subclinical hypertensive patients than in normotensive subjects. Increased levels of DNA damage, lipid peroxidation, triglyceride, total cholesterol, and LDL-cholesterol were observed in subclinical hypertensive patients. These results confirm an association between blood pressure and oxidative stress-related parameters and suggest that the pathogenic role of oxidative stress in hypertension might be significant.
Figures and Tables
Table 2
Plasma levels of antioxidant vitamins, glutathione and TRAP in the normal and hypertensive subjects
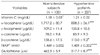
Table 6
Pearson's partial correlation coefficients between blood pressure and antioxidant-related parameters in the subjects after adjusting age, sex, body weight, body fat, BMI and WHR

References
1. Narayan KM, Ali MK, Koplan JP. Global noncommunicable diseases--where worlds meet. N Engl J Med. 2010; 363(13):1196–1198.
2. Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korea Health Statistics 2010: Korea National Health and Nutrition Examination Survey (KNHANES V-1). 2011. Cheongwon: Korea Centers for Disease Control and Prevention.
3. Harris DM, Cohn HI, Pesant S, Eckhart AD. GPCR signalling in hypertension: role of GRKs. Clin Sci (Lond). 2008; 115(3):79–89.

4. Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care. 2008; 31:Suppl 2. S181–S184.

5. Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004; 44(3):248–252.

6. Zicha J, Dobesová Z, Kunes J. Relative deficiency of nitric oxide-dependent vasodilation in salt-hypertensive Dahl rats: the possible role of superoxide anions. J Hypertens. 2001; 19(2):247–254.

7. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002; 39(2):257–265.

8. Touyz RM, Yao G, Quinn MT, Pagano PJ, Schiffrin EL. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol. 2005; 25(3):512–518.

9. Bessa SS, Ali EM, Hamdy SM. The role of glutathione S-transferase M1 and T1 gene polymorphisms and oxidative stress-related parameters in Egyptian patients with essential hypertension. Eur J Intern Med. 2009; 20(6):625–630.

10. Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res. 2011; 34(1):5–14.

11. Gur M, Yildiz A, Demirbag R, Yilmaz R, Koçyigit A, Celik H, Aksoy N. Relationship between left ventricle geometric patterns and lymphocyte DNA damage in patients with untreated essential hypertension. Clin Biochem. 2007; 40(7):454–459.

12. Chen J, He J, Hamm L, Batuman V, Whelton PK. Serum antioxidant vitamins and blood pressure in the United States population. Hypertension. 2002; 40(6):810–816.

13. Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001; 38(3 Pt 2):606–611.

14. Boshtam M, Rafiei M, Sadeghi K, Sarraf-Zadegan N. Vitamin E can reduce blood pressure in mild hypertensives. Int J Vitam Nutr Res. 2002; 72(5):309–314.

15. Han SJ, Park SH. The changes of antioxidant enzymes in the erythrocytes and placentas from patient with pregnancy-induced hypertension. Korean J Obstet Gynecol. 1996; 39(3):511–518.
16. Kim JH, Kim MJ, Kwak HK. Obesity indices and plasma total antioxidant status in hypertensive elderly living in Ulsan area. Korean J Community Nutr. 2006; 11(2):279–288.
17. Park EJ, Kim JS, Jeon EJ, Kim HY, Park YK, Kang MH. The effects of purple grape juice supplementation on improvement of antioxidant status and lymphocyte DNA damage in Korean smokers. Korean J Nutr. 2004; 37(4):281–290.
18. Lee HJ, Park YK, Kang MH. The effect of carrot juice, β-carotene supplementation on lymphocyte DNA damage, erythrocyte antioxidant enzymes and plasma lipid profiles in Korean smoker. Nutr Res Pract. 2011; 5(6):540–547.

19. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. DASH Collaborative Research Group. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997; 336(16):1117–1124.

20. Lassègue B, Griendling KK. Reactive oxygen species in hypertension; an update. Am J Hypertens. 2004; 17(9):852–860.
21. Rodrigo R, Prat H, Passalacqua W, Araya J, Guichard C, Bächler JP. Relationship between oxidative stress and essential hypertension. Hypertens Res. 2007; 30(12):1159–1167.

22. Ulker S, McKeown PP, Bayraktutan U. Vitamins reverse endothelial dysfunction through regulation of eNOS and NAD(P)H oxidase activities. Hypertension. 2003; 41(3):534–539.

23. Pezeshk A, Derick Dalhouse A. Vitamin E, membrane fluidity, and blood pressure in hypertensive and normotensive rats. Life Sci. 2000; 67(15):1881–1889.

24. Russo C, Olivieri O, Girelli D, Faccini G, Zenari ML, Lombardi S, Corrocher R. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J Hypertens. 1998; 16(9):1267–1271.

25. Tse WY, Maxwell SR, Thomason H, Blann A, Thorpe GH, Waite M, Holder R. Antioxidant status in controlled and uncontrolled hypertension and its relationship to endothelial damage. J Hum Hypertens. 1994; 8(11):843–849.
26. Miller ER 3rd, Appel LJ, Levander OA, Levine DM. The effect of antioxidant vitamin supplementation on traditional cardiovascular risk factors. J Cardiovasc Risk. 1997; 4(1):19–24.

27. Fotherby MD, Williams JC, Forster LA, Craner P, Ferns GA. Effect of vitamin C on ambulatory blood pressure and plasma lipids in older persons. J Hypertens. 2000; 18(4):411–415.

28. Block G, Mangels AR, Norkus EP, Patterson BH, Levander OA, Taylor PR. Ascorbic acid status and subsequent diastolic and systolic blood pressure. Hypertension. 2001; 37(2):261–267.

29. Palumbo G, Avanzini F, Alli C, Roncaglioni MC, Ronchi E, Cristofari M, Capra A, Rossi S, Nosotti L, Costantini C, Cavalera C. Collaborative Group of the Primary Prevention Project (PPP)--Hypertension study. Effects of vitamin E on clinic and ambulatory blood pressure in treated hypertensive patients. Am J Hypertens. 2000; 13(5 Pt 1):564–567.

30. Ward NC, Croft KD. Hypertension and oxidative stress. Clin Exp Pharmacol Physiol. 2006; 33(9):872–876.

31. Kim MK, Sasaki S, Sasazuki S, Okubo S, Hayashi M, Tsugane S. Lack of long-term effect of vitamin C supplementation on blood pressure. Hypertension. 2002; 40(6):797–803.

32. Bae I, Fan S, Meng Q, Rih JK, Kim HJ, Kang HJ, Xu J, Goldberg ID, Jaiswal AK, Rosen EM. BRCA1 induces antioxidant gene expression and resistance to oxidative stress. Cancer Res. 2004; 64(21):7893–7909.

33. Simic DV, Mimic-Oka J, Pljesa-Ercegovac M, Savic-Radojevic A, Opacic M, Matic D, Ivanovic B, Simic T. Byproducts of oxidative protein damage and antioxidant enzyme activities in plasma of patients with different degrees of essential hypertension. J Hum Hypertens. 2006; 20(2):149–155.

34. Pedro-Botet J, Covas MI, Martín S, Rubiés-Prat J. Decreased endogenous antioxidant enzymatic status in essential hypertension. J Hum Hypertens. 2000; 14(6):343–345.

35. Kasapoglu M, Ozben T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp Gerontol. 2001; 36(2):209–220.

36. Kang MH, Yun JS. The effects of exercise and other relating factors on the activity of erythrocyte antioxidant enzymes and plasma TRAP levles in male college students. Korean J Nutr. 2002; 35(1):30–36.
37. Vasconcelos SM, Goulart MO, Silva MA, Manfredini V, Benfato Mda S, Rabelo LA, Fontes G. Markers of redox imbalance in the blood of hypertensive patients of a community in Northeastern Brazil. Arq Bras Cardiol. 2011; 97(2):141–147.
38. Kesavulu MM, Rao BK, Giri R, Vijaya J, Subramanyam G, Apparao C. Lipid peroxidation and antioxidant enzyme status in Type 2 diabetics with coronary heart disease. Diabetes Res Clin Pract. 2001; 53(1):33–39.

39. Turk HM, Sevinc A, Camci C, Cigli A, Buyukberber S, Savli H, Bayraktar N. Plasma lipid peroxidation products and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Acta Diabetol. 2002; 39(3):117–122.

40. Halliwell B, Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991; 281(1-2):9–19.

41. Yildiz A, Gür M, Yilmaz R, Demirbağ R, Celik H, Aslan M, Koçyiğit A. Lymphocyte DNA damage and total antioxidant status in patients with white-coat hypertension and sustained hypertension. Turk Kardiyol Dern Ars. 2008; 36(4):231–238.
42. Lacy F, Kailasam MT, O'Connor DT, Schmid-Schonbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension. 2000; 36(5):878–884.

43. Kumar KV, Das UN. Are free radicals involved in the pathobiology of human essential hypertension? Free Radic Res Commun. 1993; 19(1):59–66.

44. Ward NC, Hodgson JM, Puddey IB, Mori TA, Beilin LJ, Croft KD. Oxidative stress in human hypertension: association with antihypertensive treatment, gender, nutrition, and lifestyle. Free Radic Biol Med. 2004; 36(2):226–232.

45. Cracowski JL, Baguet JP, Ormezzano O, Bessard J, Stanke-Labesque F, Bessard G, Mallion JM. Lipid peroxidation is not increased in patients with untreated mild-to-moderate hypertension. Hypertension. 2003; 41(2):286–288.

46. Gunes F, Akbal E, Cakir E, Akyurek O, Altunbas M, Ozbek M. Visfatin may be a novel marker for identifying stages of essential hypertension in advanced age patients. Intern Med. 2012; 51(6):553–557.

47. Zheng M, Zeng Q, Shi XQ, Zhao J, Tang CS, Sun NL, Geng B. Erythrocytic or serum hydrogen sulfide association with hypertension development in untreated essential hypertension. Chin Med J (Engl). 2011; 124(22):3693–3701.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download