Abstract
We compared the effects of grapefruit seed extract (GFSE), green tea extract (GT) and their microencapsulated extract on anti-inflammatory activities in murine RAW 264.7 macrophages cell line. In order to protect the bioactive compounds in the extracts, they were microencapsulated with maltodextrin and H2O. Nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS) protein expression and thiobarbiturate reactive substances (TBARS) were analyzed in LPS activated RAW 264.7 macrophages. The green tea extract at the range of 100-600 µg/mL inhibited NO, PGE2 production and iNOS protein expression without cytotoxicity in a dose-dependent manner. Grapefruit seed extract had strong inhibitory effects on NO and PGE production and iNOS protein expression at the range of 5-20 µg/mL without cytotoxicity. Microencapsulation of green tea extract had further inhibitory effects on NO and PGE2 production and on iNOS protein expression, whereas microencapsulated GFSE did not show any further inhibitory effects on these parameters. Taken together, our results suggest that GSFE might be a promising candidate for preventing inflammation related diseases, such as cardiovascular disease, cancer or diabetes, and the microencapsulation of green tea extract could improve its bioactivity.
Figures and Tables
 | Fig. 1Effect of green tea extract (GT), grapefruit seed extract (GSE) and microencapsulated extracts (MGT, MGSE) on cell viability in RAW 264.7 macrophages cell line. Cells were seed at initial density of 5 × 104/mL. Cells were treated with various concentrations of GT, GSE, MGT and MGSE and incubated for 48 h. GT: green tea extract, MGT: microencapsulated green tea extract, GSE: grapefruit seed extract, MGSE: microencapsulated grapefruit seed extract. |
 | Fig. 2Effect of green tea extract (GT), grapefruit seed extract (GSE) and microencapsulated extracts (MGT, MGSE) on nitric oxide Production in RAW 264.7 macrophages cell line. Cells were seed at initial density of 6 × 105/mL. Cells were treated with various concentrations of GT, GSE, MGT and MGSE and incubated for 16 h. Each colum represents the mean of triplicates. Different letters denote significant difference in nitrite level (p < 0.05). *: indicates significant difference from microencapsulated extract (p < 0.05). GT: green tea extract, MGT: microencapsulated green tea extrat, GSE: grapefruit seed extract, MGSE: microencapsulated grapefruit seed extract. |
 | Fig. 3Effect of green tea extract, grapefruit seed extract and microencapsulated extracts on prostaglandin E2 in RAW 264.7 macrophages cell line. Cells were seed at initial density of 6 × 105/mL. Cells were treated with various concentrations of GT, GSE, MGT and MGSE and incubated for 16 h. Each column represents the mean of triplicates. Different letters denote significant difference (p < 0.05). *: indicates significant difference from microencapsulated extract (p < 0.05). GT: green tea extract, MGT: microencapsulated green tea extrat, GSE: grapefruit seed extract, MGSE: microencapsulated grapefruit seed extract. |
 | Fig. 4Effect of green tea extract, grapefruit seed extract and microencapsulated extracts on tumor necrosis factor-α in RAW 264.7 macrophages cell line. Cells were seed at initial density of 6 × 105/mL. Cells were treated with various concentrations of GT, GSE, MGT and MGSE and incubated for 16 h. Each column represents the mean of triplicates. Different letters denote significant difference (p < 0.05). GT: green tea extract, MGT: microencapsulated green tea extrat, GSE: grapefruit seed extract, MGSE: microencapsulated grapefruit seed extract. |
 | Fig. 5Effect of green tea extract (GT), grapefruit seed extract (GSE) and microencapsulated extracts (MGT, MGSE) on thiobarbiturate reactive substances in RAW 264.7 macrophages cell line. Cells were seed at initial density of 5 × 105/mL. Cells were treated with various concentrations of GT, GSE, MGT and MGSE and incubated for 16 h. Each column represents the mean of triplicates. Different letters denote significant difference (p < 0.05). GT: green tea extract, MGT: microencapsulated green tea extract, GSE: grapefruit seed extract, MGSE: microencapsulated grapefruit seed extract. |
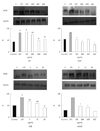 | Fig. 6Effect of green tea extract (GT), grapefruit seed extract (GSE) and microencapsulated extracts (MGT, MGSE) on expression of inducible nitric oxide synthase (iNOS) in RAW 264.7 macrophages cell line. Cells were seed in 100 mm dish at initial density of 5 × 106/mL. Cells were treated with various concentrations of GT, MGT, GSE, MGSE and incubated for 16 h. Each column represents the mean of triplicates. Different letters denote significant difference (p < 0.05). *: indicates significant difference from microencapsulated extract (p < 0.05). GT: green tea extract, MGT: microencapsulated green tea extract, GSE: grapefruit seed extract, MGSE: microencapsulated grapefruit seed extract. |
References
2. Byers T, Perry G. Dietary carotenes, vitamin C, and vitamin E as protective antioxidants in human cancers. Annu Rev Nutr. 1992. 12:139–159.

3. Bogdan C, Röllinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev. 2000. 173(1):17–26.

4. Evans CH. Nitric oxide: what role does it play in inflammation and tissue destruction? Agents Actions Suppl. 1995. 47:107–116.

5. Grosjean SA, Arstall MA, Mitchell RN, Klappacher GW, Kelly RA, Pfeffer MA, Pfeffer JM. Inducible nitric oxide synthase and tumor necrosis factor in animal models of myocardial necrosis induced by coronary artery ligation or isoproterenol injection. J Card Fail. 1999. 5(3):236–245.

6. Crook MA, Tutt P, Pickup JC. Elevated serum sialic acid concentration in NIDDM and its relationship to blood pressure and retinopathy. Diabetes Care. 1993. 16(1):57–60.

7. Nathan CF, Hibbs JB Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991. 3(1):65–70.

8. Tang SY, Whiteman M, Jenner A, Peng ZF, Halliwell B. Mechanism of cell death induced by an antioxidant extract of Cratoxylum cochinchinense (YCT) in Jurkat T cells: the role of reactive oxygen species and calcium. Free Radic Biol Med. 2004. 36(12):1588–1611.

9. Ebrahimzadeh MA, Pourmorad F, Bekhradnia AR. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr J Biotechnol. 2008. 7(18):3188–3192.
10. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993. 342(8878):1007–1011.

11. Mirmiran P, Noori N, Zavareh MB, Azizi F. Fruit and vegetable consumption and risk factors for cardiovascular disease. Metabolism. 2009. 58(4):460–468.

12. Proteggente AR, Pannala AS, Paganga G, Van Buren L, Wagner E, Wiseman S, Van De Put F, Dacombe C, Rice-Evans CA. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic Res. 2002. 36(2):217–233.

13. Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health. J Sci Food Agric. 2000. 80(12):1744–1756.

14. Al-Saikhan MS, Howard LR, Miller JC. Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum, L.). J Food Sci. 1995. 60(2):341–343.

15. Vinson JA, Su X, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem. 2001. 49(11):5315–5321.

16. Singh R, Akhtar N, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate: inflammation and arthritis. [corrected]. Life Sci. 2010. 86(25-26):907–918.

17. Serafini M, Ghiselli A, Ferro-Luzzi A. In vivo antioxidant effect of green and black tea in man. Eur J Clin Nutr. 1996. 50(1):28–32.
18. Conney AH, Wang ZY, Huang MT, Ho CT, Yang CS. Inhibitory effect of green tea on tumorigenesis by chemicals and ultraviolet light. Prev Med. 1992. 21(3):361–369.

19. Ishikawa T, Suzukawa M, Ito T, Yoshida H, Ayaori M, Nishiwaki M, Yonemura A, Hara Y, Nakamura H. Effect of tea flavonoid supplementation on the susceptibility of low-density lipoprotein to oxidative modification. Am J Clin Nutr. 1997. 66(2):261–266.

20. Keevil JG, Osman HE, Reed JD, Folts JD. Grape juice, but not orange juice or grapefruit juice, inhibits human platelet aggregation. J Nutr. 2000. 130(1):53–56.

21. Yue X, Xu Z. Changes of anthocyanins, anthocyanidins, and antioxidant activity in bilberry extract during dry heating. J Food Sci. 2008. 73(6):C494–C499.

23. Davies AJ, Mazza G. Copigmentation of simple and acylated anthocyanins with colorless phenolic-compounds. J Agric Food Chem. 1993. 41(5):716–720.

24. Main JH, Clydesdale FM, Francis FJ. Spray drying anthocyanin concentrates for use as food colorants. J Food Sci. 1978. 43(6):1693–1694.

25. Ersus S, Yurdagel U. Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. J Food Eng. 2007. 80(3):805–812.

26. Takahashi M, Uechi S, Takara K, Asikin Y, Wada K. Evaluation of an oral carrier system in rats: bioavailability and antioxidant properties of liposome-encapsulated curcumin. J Agric Food Chem. 2009. 57(19):9141–9146.

27. Lee HD, Kim HS, Seong PN, Kim MH. Effect of microencapsulation processing on functional property of natural pigment and antioxidant. Food Eng Prog. 2012. 16(3):283–286.
28. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65(1-2):55–63.

29. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982. 126(1):131–138.

30. Radkar V, Hardej D, Lau-Cam C, Billack B. Evaluation of resveratrol and piceatannol cytotoxicity in macrophages, T cells, and skin cells. Arh Hig Rada Toksikol. 2007. 58(3):293–304.

31. Smith JR, Hart PH, Williams KA. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol Cell Biol. 1998. 76(6):497–512.

32. Chiou WF, Chen CF, Lin JJ. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. Br J Pharmacol. 2000. 129(8):1553–1560.

33. Tsai PJ, Tsai TH, Yu CH, Ho SC. Comparison of NO-scavenging and NO-suppressing activities of different herbal teas with those of green tea. Food Chem. 2007. 103(1):181–187.

34. Nakagawa T, Yokozawa T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem Toxicol. 2002. 40(12):1745–1750.

35. Paquay JB, Haenen GR, Stender G, Wiseman SA, Tijburg LB, Bast A. Protection against nitric oxide toxicity by tea. J Agric Food Chem. 2000. 48(11):5768–5772.

36. Mandadi KK, Jayaprakasha GK, Bhat NG, Patil BS. Red Mexican grapefruit: a novel source for bioactive limonoids and their antioxidant activity. Z Naturforsch C. 2007. 62(3-4):179–188.

37. Kaur M, Agarwal CH, Agarwal R. Anticancer and cancer chemopreventive potential of grape seed extract and other grpaebased product. J Nutr. 2009. 139:1806S–1812S.
38. Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilites of vitamin C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathos Pharmacol. 1997. 95:179–189.
39. Pedrielli P, Pedulli GF, Skibsted LH. Antioxidant mechanism of flavonoids. Solvent effect on rate constant for chain-breaking reaction of quercetin and epicatechin in autoxidation of methyl linoleate. J Agric Food Chem. 2001. 49(6):3034–3040.

40. Yu J. Citrus limonoids and flavonoids: extraction, antioxidant activity and effects on hamster plasma cholesterol distribution [dissertation]. 2004. College Station (TX): Texas A & M University.
41. Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell cultrue and in vivo studies? Arc Biochem Biophys. 2008. 476:107–112.

42. McClements DJ. Design of nano-laminated coatings to control bioavailability of lipophilic food components. J Food Sci. 2010. 75(1):R30–R42.

43. Fang Z, Bhandari B. Encapsulation of polyphenols-a review. Trends Food Sci Technol. 2010. 21(10):510–523.
44. Zhang L, Mou D, Du Y. Procyanidins: extraction and micro-encapsulation. J Sci Food Agric. 2007. 87(12):2192–2197.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download