Abstract
Figures and Tables
 | Fig. 1Acute metabolic effects by various forms of calcium supplement. NC: normal control, CD-C: Vehicle administration on calcium deficient rat, CD-CaCO3: CaCO3 administration on calcium deficient rat, CD-AAC: amino acid chelated calcium (AAC) administration on calcium deficient rat, CD-SWC: seaweed-derived calcium administration on calcium deficient rat. Bloods were collected in two hours following first administration. Values are Mean ± SD for 6 rats. Means with different superscript letters are significantly different at p < 0.05 by Duncan's multiple range tests. |
 | Fig. 2Changes of length (A) and weight (B) on fibula by various forms of calcium supplement. NC: normal control, CD-C: Vehicle administration on calcium deficient rat, CD-CaCO3: CaCO3 administration on calcium deficient rat, CD-AAC: amino acid chelated calcium (AAC) administration on calcium deficient rat, CD-SWC: seaweed-derived calcium administration on calcium deficient rat. Fibulas were collected in 7 days following first administration. Values are Mean ± SD for 6 rats. Means with different superscript letters are significantly different at p < 0.05 by Duncan's multiple range tests. ns: not significant. |
Table 1

1)NC (normal control) group: AIN-76 mineral mixture, CD (calcium Deficient) groups: Ca deficient mineral mixture 2)Vitamin mixture: AIN-93G.
NC: normal control, CD-C: vehicle administration on calcium deficient rat, CD-CaCO3: CaCO3 administration on calcium deficient rat, CD-AAC: amino acid chelated calcium (AAC) administration on calcium deficient rat, CD-SWC: seaweed-derived calcium administration on calcium deficient rat
Table 2
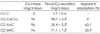
CD-C: vehicle administration on calcium deficient rat, CD-CaCO3: CaCO3 administration on calcium deficient rat, CD-AAC: amino acid chelated calcium (AAC) administration on calcium deficient rat, CD-SWC: seaweed-derived calcium administration on calcium deficient rat. Feces were collected for 3 days following first administration. Values are Mean ± SD for 6 rats. Means with different superscript letters in Ca intake group are significantly different at p < 0.05 by Duncan's multiple range tests
Table 3

1)IBW: Initial body weight 2)FBW: Final body weight 3)TDI: Total diet intake. Values are Mean ± SD for 6 rats. Means with different superscript letters in same items are significantly different at p < 0.05 by Duncan's multiple range tests. ns: not significant
NC: normal control, CD-C: vehicle administration on calcium deficient rat, CD-CaCO3: CaCO3 administration on calcium deficient rat, CD-AAC: amino acid chelated calcium (AAC) administration on calcium deficient rat, CD-SWC: seaweed-derived calcium administration on calcium deficient rat
Table 4

NC: normal control, CD-C: vehicle administration on calcium deficient rat, CD-CaCO3: CaCO3 administration on calcium deficient rat, CD-AAC: amino acid chelated calcium (AAC) administration on calcium deficient rat, CD-SWC: seaweed-derived calcium administration on calcium deficient rat. Values are Mean ± SD for 6 rats. Means with different superscript letters in same items are significantly different at p < 0.05 by Duncan's multiple range tests. ns: not significant




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download