Abstract
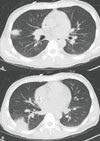
Voriconazole is a triazole with broad spectrum antifungal activity, and it is currently considered to be the first-line agent for the treatment of invasive aspergillosis. We report here on a case of visual and auditory hallucinations during intravenous treatment with voriconazole in association with a high trough level. A 28-year-old man with acute myelogenous leukemia was admitted for re-induction remission chemotherapy. During the persistent neutropenic fever, intravenous voriconazole was administered for the suspected invasive fungal pneumonia. He began to have visual hallucinations on the 1st day and auditory hallucinations on the 3rd day of voriconazole therapy. The plasma peak and trough concentration levels of voriconazole were 9.9 and 7.4 µg/ml, respectively, on the 3rd day. The hallucinations resolved after changing to amphotericin B deoxycholate, and the plasma concentration of voriconazole dropped to less than 0.5 µg/ml. The genotype of the CYP2C19 alleles was classified as a heterozygous extensive metabolizer. We suggest that therapeutic drug monitoring of voriconazole is indicated for a case that is suspicious for a voriconazole-related adverse event.
Figures and Tables
References
1. Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, dePauw B. Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002. 347:408–415.

2. Boyd AE, Modi S, Howard SJ, Moore CB, Keevil BG, Denning DW. Adverse reactions to voriconazole. Clin Infect Dis. 2004. 39:1241–1244.

3. Kwon JC, Kim SH, Choi SM, Choi JK, Lee DG, Park SH, Choi JH, Yoo JH, Shin WS. Efficacy and safety profile of voriconazole as salvage therapy for invasive aspergillosis with hematologic diseases in Korea. Infect Chemother. 2010. 42:17–22.

4. Zonios DI, Gea-Banacloche J, Childs R, Bennett JE. Hallucinations during voriconazole therapy. Clin Infect Dis. 2008. 47:e7–e10.

5. Miyakis S, van Hal SJ, Ray J, Marriott D. Voriconazole concentrations and outcome of invasive fungal infections. Clin Microbiol Infect. 2010. 16:927–933.

6. Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008. 46:201–211.

7. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981. 30:239–245.

8. Briefing document for voriconazole. US Food and Drug Administration. Accessed 15 July 2008. Available at: http://www.fda.gov/ohrms/dockets/ac/01/briefing/3792b2_01_Pfizer.pdf.
9. Brüggemann RJ, Donnelly JP, Aarnoutse RE, Warris A, Blijlevens NM, Mouton JW, Verweij PE, Burger DM. Therapeutic drug monitoring of voriconazole. Ther Drug Monit. 2008. 30:403–411.

10. Agrawal AK, Sherman LK. Voriconazole-induced musical hallucinations. Infection. 2004. 32:293–295.

11. Pasqualotto AC, Xavier MO, Andreolla HF, Linden R. Voriconazole therapeutic drug monitoring: focus on safety. Expert Opin Drug Saf. 2010. 9:125–137.

12. Ikeda Y, Umemura K, Kondo K, Sekiguchi K, Miyoshi S, Nakashima M. Pharmacokinetics of voriconazole and cytochrome P450 2C19 genetic status. Clin Pharmacol Ther. 2004. 75:587–588.

13. Shi HY, Yan J, Zhu WH, Yang GP, Tan ZR, Wu WH, Zhou G, Chen XP, Ouyang DS. Effects of erythromycin on voriconazole pharmacokinetics and association with CYP2C19 polymorphism. Eur J Clin Pharmacol. 2010. 66:1131–1136.

14. Roh HK, Dahl ML, Tybring G, Yamada H, Cha YN, Bertilsson L. CYP2C19 genotype and phenotype determined by omeprazole in a Korean population. Pharmacogenetics. 1996. 6:547–551.

15. Sohn YH, Lee WS, Park CH, Joo YE, Kim HS, Choi SK, Rew JS, Kim SJ. Influence of CYP2C19 polymorphism and helicobacter pylori status on the antisecretory effect of omeprazole in gastroesophageal reflux disease. Korean J Gastroenterol. 2006. 48:162–171.
16. Lee JH, Jung HY, Choi KD, Song HJ, Lee GH, Kim JH. The influence of CYP2C19 polymorphism on eradication of helicobacter pylori: a prospective randomized study of lansoprazole and rabeprazole. Gut Liver. 2010. 4:201–206.

17. Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos. 2003. 31:540–547.

18. Blume H, Donath F, Warnke A, Schug BS. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. 2006. 29:769–784.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download