Abstract
Libman-Sacks endocarditis (LSE) is a valvular heart disease that is associated with autoimmune diseases such as systemic lupus erythematosus and antiphospholipid syndrome (APS). Cases of LSE and APS associated with infection have been reported during the last several years. Herein, we present a patient who was suspected to have developed LSE and catastrophic APS during the treatment of her definite infective endocarditis, which was caused by Staphylococcus aureus, and the patient's condition was complicated with cerebral abscess, sensorineural hearing loss, endophthalmitis, renal infarction, splenic abscess, and septic arthritis.
Libman-Sacks endocarditis (LSE) is a cardiac manifestation of antiphospholipid syndrome (APS), usually presents as nonbacterial endocarditis [1]. One of the triggering conditions of APS is infectious diseases such as skin infections, human immunodeficiency virus infection, pneumonia, hepatitis C virus infections, and urinary tract infections [2]. We describe here a case of a 38-year-old woman with Staphylococcus aureus infective endocarditis (IE) and multiple systemic embolisms. The patient was diagnosed as having developed LSE during the antimicrobial treatment.
A 38-year-old woman was referred to our hospital with symptoms of fever and severe headache that had lasted for the previous 4 days. She had a history of intermittent epileptic seizures 8 years ago and she was taking lamotrigine and folic acid. In recent years, she had experienced several episodes of aura, but without seizures.
In the emergency department, her body temperature was 38.1℃, blood pressure was 100.63 mmHg, pulse rate was 110/min, and respiratory rate was 17/min. On physical examination, she showed neck stiffness with positive Kernig and Brudzinski signs. There were multiple small-sized pustules on the face and several 5-10 mm sized, nodular skin lesions on both lower extremities, which were considered to be Janeway lesions and Osler's nodes (Fig. 1).
The white blood cell count was 10,050/mm3. The hemoglobin level was 9.2 g/dL, and the platelet count was 107,000/mm3. The other blood chemistry results were not remarkable. The cerebrospinal fluid (CSF) was colorless and clear. The CSF WBC was 143/mm3, of which 90% were polymorphonuclear cells. CSF protein was 64.3 mg/dL and CSF glucose was 65 mg/dL. We started empirical vancomycin 2 g/day and ceftriaxone 2 g/day intravenously.
Her abdominal computed-tomographic scan showed multiple microabscesses in the spleen with splenomegaly (about 13 cm in size) and septic infarction on both kidneys (Fig. 2). Cerebral magnetic resonance imaging (MRI) showed multifocal brain abscesses due to septic embolism (Fig. 3). However, there was no evidence of endocardial infection on both the trans-thoracic (TTE) and trans-esophageal (TEE) echocardiograms.
On day 4, methicillin-sensitive S. aureus was isolated from 2 of 3 blood cultures and the CSF. The diagnosis of definite IE was made according to the modified Duke Criteria (1 major and 3 minor criteria). We replaced vancomycin and ceftriaxone with nafcillin 12 g/day. However, she started to experience newly-developed knee and shoulder pain. Synovial fluid analysis of the knee showed a WBC count of 57,200/mm3, a glucose level of 89 mg/dL, and negativity for mosodium urate crystals.
On day 8, she complained of an inflamed left eye with loss of eyesight. She was diagnosed as having endogenous endophthalmitis according to an ophthalmoscope examination.
On day 20, all of the IE-related symptoms, including fever, headache, diplopia, and arthralgia, were improved. However, follow-up TEE revealed a new valvular lesion on the mitral valve, and this was suggestive of non-bacterial thrombi rather than infective vegetation (Fig. 4). Interestingly, the lupus anticoagulant (LAC) assay showed a positive result. Additional laboratory tests showed negativity for anti-nuclear and anti-double-stranded DNA antibodies and normal serum levels of anti-cardiolipin and anti-beta 2 glycoprotein I antibodies. Anticoagulation with warfarin was instituted for the treatment of possible APS.
After three weeks of anticoagulation, follow-up TTE did not show any intracardiac vegetation. She was discharged from the hospital after completion of the antimicrobial treatment. Anticoagulation treatment was continued for 3 months at the outpatient clinic. The LAC assay repeated 3 months later showed a positive result.
Systemic embolic events occur in 22-68% of the patients with IE, and more than 50% of the embolic episodes involve the central nervous system [3]. The risk factors for developing emboli in patients with IE include staphylococcal infection, mitral valve disease, and the presence of large cardiac vegetation [3]. Although the TEE of our patient revealed no valvular lesions at the time of admission, she was considered to be at a high risk for embolism with IE because she had a staphylococcal infection.
Thrombophilic conditions such as antiphospholipid antibodies, abnormal coagulation parameters, and endothelial cell activation are also associated with an increased embolic risk in patients with IE [4]. On follow-up TEE of our patient, there was a newlydeveloped, mass-like lesion at the mitral valve, which was firmly attached to the valve surface. It exhibited no independent motion. It looked like non-bacterial vegetation, the same as reported in previous case studies [5, 6]. Therefore, she was suspected to have a thrombophilic condition, and was found to have APS. APS was confirmed by consecutive positive LAC performed 12 weeks apart, which fits in with the current diagnostic criteria [7]. She had a healthy child and denied any previous history of recurrent fetal loss, which suggested that her APS probably developed after the IE.
Anticoagulation is usually not a routine treatment for patients with IE. However, the current therapeutic guidelines for APS include thrombo embolic prevention with long-term anticoagulation [1]. In our case, cerebral MR angiography was performed prior to anticoagulation and no cerebral mycotic aneurysm was found.
LSE was first described by Libman and Sacks in 1924 in three patients with lupus [6]. The association between LSE and APS was noted in 1985 and additional evidence has been reported that antiphospholipid antibodies are related to the pathogenesis of valvular heart disease [8-10]. On the other hand, several studies have suggested the association of APS/LSE and infection [2, 11, 12]. Cervera et al. reported that various infections could be related to thrombotic events in patients with APS, and that catastrophic APS, which is the most severe form of APS, seemed to be triggered mainly by infections [2, 13]. Although there have been episodic reports of LSE without infection in Korea [14, 15], there has been no reported Korean case of LSE combined with infection. Our patient could be diagnosed with "probable" catastrophic APS based on the proposed preliminary classification criteria in 2002, because she had multiple organs involved that developed within 1 week, the laboratory confirmation of LAC, and the lack of pathological confirmation of thrombosis [16]. However, we could not conclude whether the probable catastrophic APS or staphylococcal IE itself was the main cause of multifocal emboli in our patient due to the lack of histologic information.
In conclusion, we have described a case of LSE and APS and this all developed after staphylococcal IE with cerebral abscess, sensorineural hearing loss, endophthalmitis, renal infarction, splenic abscess, and septic arthritis. To the best of our knowledge, such an unusual presentation of LSE has not been reported so far in Korea. We suggest that serial echocardiography may be needed in cases of IE combined with systemic emboli, and if the echocardiography shows unusual or newly-developed vegetation during the treatment of IE, then a clinical suspicion and proper evaluation of APS are mandatory.
Figures and Tables
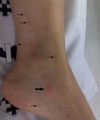 | Figure 1Multiple small Janeway lesions (narrow arrow) and one Osler node (thick arrow) are seen on the medial side of the left leg. |
 | Figure 2The abdomen computed tomographic scan shows multiple renal infarctions (A) and splenic microabscesses with splenomegaly (about 13 cm in size) (B). |
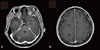 | Figure 3Cerebral magnetic resonance imaging reveals multifocal brain abscesses. The lesions of rim enhancement with central necrosis on the enhanced T1-weighted image are shown in the cerebellum (A) and the right hemisphere (B). |
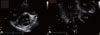 | Figure 4Transthoracic echocardiography shows a mobile, round, heterogeneous echogenic material with a smooth surface at the posterior mitral valve, which exhibits no independent motion (A). Transesophageal echocardiography reveals a 1.5 × 0.9 cm sized oval mass located on the atrial side of the posterior mitral leaflet (B).
RV, right ventricle; LA, left atrium; PA, pulmonary artery; LV, left ventricle; RA, right atrium
|
References
1. Hojnik M, George J, Ziporen L, Shoenfeld Y. Heart valve involvement (Libman-Sacks endocarditis) in the antiphospholipid syndrome. Circulation. 1996. 93:1579–1587.

2. Cervera R, Asherson RA, Acevedo ML, Gómez-Puerta JA, Espinosa G, De La Red G, Gil V, Ramos-Casals M, García-Carrasco M, Ingelmo M, Font J. Antiphospholipid syndrome associated with infections: clinical and microbiological characteristics of 100 patients. Ann Rheum Dis. 2004. 63:1312–1317.

3. Hsu RB, Lin FY. Methicillin resistance and risk factors for embolism in Staphylococcus aureus infective endocarditis. Infect Control Hosp Epidemiol. 2007. 28:860–866.

4. Kupferwasser LI, Hafner G, Mohr-Kahaly S, Erbel R, Meyer J, Darius H. The presence of infection-related antiphospholipid antibodies in infective endocarditis determines a major risk factor for embolic events. J Am Coll Cardiol. 1999. 33:1365–1371.

5. Bouma W, Klinkenberg TJ, van der Horst IC, Wijdh-den Hamer IJ, Erasmus ME, Bijl M, Suurmeijer AJ, Zijlstra F, Mariani MA. Mitral valve surgery for mitral regurgitation caused by Libman-Sacks endocarditis: a report of four cases and a systematic review of the literature. J Cardiothorac Surg. 2010. 5:13.

6. Libman E, Sacks B. A hitherto undescribed form of valvular and mural endocarditis. Arch Intern Med. 1924. 33:701–737.

7. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006. 4:295–306.

8. D'Alton JG, Preston DN, Bormanis J, Green MS, Kraag GR. Multiple transient ischemic attacks, lupus anticoagulant and verrucous endocarditis. Stroke. 1985. 16:512–514.
9. Asherson RA, Hughes GR. The expanding spectrum of Libman Sacks endocarditis: the role of antiphospholipid antibodies. Clin Exp Rheumatol. 1989. 7:225–228.
10. Khamashta MA, Gil A, Asherson RA, Vazquez JJ, Hughes GR. Antiphospholipid antibodies, valvular heart disease and systemic lupus erythematosus. Am J Med. 1989. 86:633–634.
11. Blank M, Shani A, Goldberg I, Kopolovic J, Amigo MC, Magrini L, Shoenfeld Y. Libman-Sacks endocarditis associated with antiphospholipid syndrome and infection. Thromb Res. 2004. 114:589–592.

12. Zinger H, Sherer Y, Goddard G, Berkun Y, Barzilai O, Agmon-Levin N, Ram M, Blank M, Tincani A, Rozman B, Cervera R, Shoenfeld Y. Common infectious agents prevalence in antiphospholipid syndrome. Lupus. 2009. 18:1149–1153.

13. Cervera R, Bucciarelli S, Plasín MA, Gómez-Puerta JA, Plaza J, Pons-Estel G, Shoenfeld Y, Ingelmo M, Espinos G. Catastrophic Antiphospholipid Syndrome (CAPS) Registry Project Group (European Forum On Antiphospholipid Antibodies). Catastrophic antiphospholipid syndrome (CAPS): descriptive analysis of a series of 280 patients from the "CAPS Registry". J Autoimmun. 2009. 32:240–245.

14. Kim JY, Choi SH, Hong GR, Kang SM, Park YB, Rim SJ, Chung N. A case of Libman-Sacks endocarditis with moderate mitral regurgitation. Korean Circ J. 2003. 33:715–718.

15. Rhee SY, Sohn IS, Kim SJ, Kang HS, Choue CW, Song JS, Bae JH. A case of Libman-Sacks endocarditis confused with infective endocarditis. Korean J Med. 2004. 67:89–93.
16. Asherson RA, Cervera R, de Groot PG, Erkan D, Boffa MC, Piette JC, Khamashta MA, Shoenfeld Y. Catastrophic Antiphospholipid Syndrome Registry Project Group. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003. 12:530–534.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download