Abstract
We previously demonstrated that in vivo engagement of CD137, a member of TNF receptor superfamily, can delete allorective CD4+ T cells through the induction of activation-induced cell death (AICD) in chronic graft-versus-host disease (cGVHD) and subsequently reverse established cGVHD. In this study, we further showed that agonistic anti-CD137 mAb was highly effective in triggering AICD of donor CD8+ T cells as well as donor CD4+ T cells in the C57BL/6→unirradiated (C57BL/6 × DBA/2)F1 acute GVHD model. Our results suggest that strong allostimulation should facilitate AICD of both alloreactive CD4+ and CD8+ T cells induced by CD137 stimulation. Therefore, depletion of pathogenic T cells using agonistic anti-CD137 mAb combined with potent TCR stimulation may be used to block autoimmune or inflammatory diseases mediated by T cells.
CD137 is a member of the TNF receptor family and functions as a costimulatory molecule for T cells (1). Engagement of CD137 using anti-CD137 mAbs has been shown to effectively eradicate established tumors mainly through activation of CD8+ T cells (1). Paradoxically, however, anti-CD137 mAbs have strong immunosuppressive effects on a variety of autoimmune or inflammatory diseases that are believed to be mediated mainly by CD4+ T cells (2). A consensus on how stimulation of CD137 prevents disease has yet to emerge, but CD137 appears to be involved in the hyperactivation of T cells, causing them to acquire regulatory capacity or induce cell death (3).
In the DBA/2→unirradiated (C57BL/6 × DBA/2)F1 (BDF1) chronic graft-versus-host disease (cGVHD) model, anti-CD137 mAb is highly effective in inhibiting cGVHD by deleting donor CD4+ T cells which are required for breaking host B-cell tolerance (4). In a more clinically relevant cGVHD model, anti-CD137 mAb reverses skin fibrosis, ulceration, and alopecia, a dominant feature of cGVHD, ultimately improving a general health condition (5). The reversal is associated with increased apoptosis of donor CD4+ T cells. The Fas death pathway is required for activation-induced cell death (AICD) of alloreactive CD4+ T cells.
In this study, we hypothesized that anti-CD137 mAb might induce AICD of alloreactive CD8+ T cells as well as CD4+ T cells if they received strong allostimulation. We chose the C57BL/6→unirradiated BDF1 acute GVHD (aGVHD) model as a model system to test this hypothesis, since strong alloimmunity for donor CD4+ and CD8+ T cells occurs in this disease model. BDF1 mice received C57BL/6 T cells and anti-CD137 mAb (3H3) immediately after the cell transfer. FACS analysis showed that there was a marked increase in apoptosis of both splenic CD4+ and CD8+ T cells in anti-CD137-treated mice 5 days after the cell transfer (Fig. 1A). A majority of donor CD4+ and CD8+ T cells expressed low levels of CD62L in both control Ig- and anti-CD137-treated mice (Fig. 1B), indicating that AICD caused their apoptosis following injection of anti-CD137 mAb, as seen previously (4). At this time point, a higher percent of donor CD4+T cells underwent activation and apoptosis following administration of anti-CD137 mAb, as compared with donor CD8+ T cells (Fig. 1). This result may indicate that donor CD4+ T cells had a more rapid kinetics for their activation and subsequent AICD in response to anti-CD137 mAb.
A long-term observation demonstrated that control Ig-treated mice experienced severe loss of body weight and their mortality rate was high (70%), whereas anti-CD137-treated mice maintained normal body weight and stayed healthy until the termination of experiments (Fig. 2). FACS analysis for splenocytes showed that administration of anti-CD137 mAb prevented severe lymphodepletion in the spleen, a parameter for aGVHD (Table I). Anti-CD137-mediated inhibition of aGVHD was due to the deletion of donor CD4+ and CD8+ T cells and a subsequent failure of donor cell engraftment (Table I).
In two preclinical models of cGVHD, now it is clear that agonistic anti-CD137 mAb has the ability to delete pathogenic alloreactive CD4+ T cells and autoreactive B cells. However, there is evidence showing that agonistic anti-CD137 mAb can delete antigen-specific CD8+ T cells as well as CD4+ T cells in vivo(6,7). Earlier treatment with agonistic anti-CD137 mAb maintains elevated levels of TNF-α in lymphocytic choriomeningitis virus (LCMV)-infected mice, leading to Fas expression on activated CD8+T cells and this in turn results in Fas-mediated apoptosis (6). Even though Fas-mediated death signal is not sufficient to delete LCMV antigen-specific CD8+ T cells, STAT3 activation by signaling through CD137 in dendritic cells is required for their complete AICD (7). In this study, we showed that agonistic anti-CD137 mAb could completely delete not only donor CD4+ T cells but also CD8+ T cells in the C57BL/6→BDF1 aGVHD model. As seen in cGVHD (4,5) it is likely that engagement of CD137 provides strong costimulatory signaling leading to AICD for donor CD4+and CD8+ T cells that receive sustained allostimulation during the evolution of aGVHD. It remains to be elucidated whether other host hematopoietic and/or nonhematopoietic cells are needed for AICD of donor T cells by agonistic anti-CD137 mAb in GVHD.
Figures and Tables
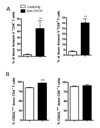 | Figure 1Anti-CD137 mAb induces apoptosis of both donor CD4+ and CD8+ T cells in aGVHD. aGVHD was induced by transferring 5×107 C57BL/6 spleen/lymph node cells into BDF1 mice. Immediately thereafter, anti-CD137 mAb or control Ig (200µg per mouse) was injected. Splenocytes were analyzed by flow cytometry 5 days after parental cell transfer. (A) Percent of Annexin V+ donor CD4+ and CD8+ T cells. (B) Percent of CD62Llow donor CD4+ and CD8+ T cells. n=3 mice per group. *p<0.01 and ***p<0.001 between the 2 groups. |
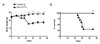 | Figure 2Anti-CD137 mAb completely blocks aGVHD. aGVHD was induced by transferring 5×107 C57BL/6 spleen/lymph node cells into BDF1 mice. Immediately thereafter, anti-CD137 mAb or control Ig (200µg per mouse) was injected (n=10 per group). (A) Changes of body weight. *p<0.05 and ***p<0.001 between the 2 groups at the indicated time points. (B) Survival curve. **p<0.05 between the 2 groups. |
Table I
Inhibition of donor cell engraftment by anti-CD137 mAba

aaGVHD was induced by transferring 5×107 C57BL/6 spleen/lymph node cells into BDF1 mice. Immediately theafter, mice received control lg or anti-CD137 mAb (200 µg per mouse). Splenocytes were analyzed by flow cytometry 44 days after disease induction. Absolute number or percent of donor cells were counted by staining splenocytes with anti-H-2Kb plus anti-B220, anti-CD4 or anti-CD8 mAbs. bValues for total splenocytes and lymphocyte subsets are shown as (mean±SD)×10-6 (n=10 mice per group).
ACKNOWLEDGEMENTS
This work was supported by Grant 20090094052 from the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology.
References
2. Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997. 3:682–685.

3. Kwon B. Intervention with costimulatory pathways as a therapeutic approach for graft-versus-host disease. Exp Mol Med. 2010. 42:675–683.

4. Kim J, Choi WS, La S, Suh JH, Kim BS, Cho HR, Kwon BS, Kwon B. Stimulation with 4-1BB (CD137) inhibits chronic graft-versus-host disease by inducing activation-induced cell death of donor CD4+ T cells. Blood. 2005. 105:2206–2213.

5. Kim J, Kim HJ, Park K, Kim J, Choi HJ, Yagita H, Nam SH, Cho HR, Kwon B. Costimulatory molecule-targeted immunotherapy of cutaneous graft-versus-host disease. Blood. 2007. 110:776–782.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download