Mycobacterium leprae, the causative agent of leprosy, is an intracellular pathogen that often resides within specialized compartments and replicates in macrophages. This pathogen can induce macrophages to release inflammatory cytokines, such as IL-1β, IL-12, and TNF-α which are involved in the innate immune response for bacterial elimination and the coordination of adaptive immune responses (1-3).
Pattern recognition receptors (PRRs) are essential components for recognizing conserved microbial structure known as pathogen-associated molecular patterns (PAMPs) and stimulating production of pro-inflammatory cytokines in the innate immune system. There are three major classes of PRRs; toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), and nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) (4). Among these, NLRs are intracellular and cytoplasmic sensors that involved in several biological processes, which include host defense against pathogen and inflammation (5,6).
Stimulation of NOD1 and NOD2, which are a subfamily of NLRs, leads to activation of the nuclear transcription factor (NF)-κB and AP-1, p38, extracellular signal-regulated kinase (ERK), and Jun N-terminal kinase (JNK), which are known to be triggered by mycobacteria (7,8). These activations of intracellular pathways result in expression of pro-inflammatory molecules that arouse both innate and adaptive immune responses. Both NOD1 and NOD2 sense bacterial molecules produced during the synthesis and degradation of peptidoglycan. NOD2 is especially activated by muramyl dipeptide (MDP), a component of peptidoglycan (PGN) (9-11). NOD2 has also been implicated in sensing intracellular pathogens such as Listeria monocytogenes (7) and Mycobacterium tuberculosis (12). However, despite their importance, the roles of NOD 1 and NOD 2 have not been elucidated in M. leprae infection. The role of NOD1 and NOD2 on the activation of NF-κB in response to M. leprae was determined in this study.
M. leprae was prepared from the foot-pads of M. leprae-infected nude mice as previously described (3) and bacteria were counted by the procedure of Shepard and McRae (13). The human embryonic kidney (HEK) 293 T cells and mouse RAW 264.7 cell lines were purchased from American Type Culture Collection. The cell lines were cultivated in DMEM (Hyclone) media supplemented with 10% fetal bovine serum (Hyclone) and 1% penicillin-streptomycin (Gibco).
Raw274.7 cells were infected for 8 h with M. leprae at a multiplicity-of-infection (MOI) of 1 or 10. In some experiments, the cells were incubated for 30 min with cytochalasin D, a compound that inhibits phagocytosis by interacting with microfilament. Culture supernatants were assayed for mouse IL-1β, IL-6, IL-12, and TNF-α by ELISA (DuoSet, R & D) according to the manufacturer's protocol.
HEK293T cells were seeded into 12-well Falcon plates (BD Falcon) at 2×105 cells/well, and then incubated overnight in a CO2 incubator. Cells were washed with DPBS and co-transfected for 3 hr with varying amounts of pcDNA3-NOD1 and pcDNA3-NOD2. The final DNA quantity was adjusted to 1.5 µg/well with empty pcDNA3 vector (Invitrogen, Carlsbad, CA). The transfection was carried out with FuGENE HD transfection reagent (Roche) according to the manufacturer's protocol. Cells were washed with DPBS and left overnight in a CO2 incubator in DMEM. The transfected cells were washed with DPBS placed in serum-free DMEM and stimulated with M. leprae at an M.O.I. of 10.
Cytosolic and nuclear extracts were isolated and NF-κB activity assay was performed by the colorimetric assay kit (NF-κB EZ-TFA Transcription Factor Assay, Upstate) according to the manufacturer's instructions.
Total RNA from M. leprae-infected HEK293T cells was prepared using Trizol reagent (Invitrogen) and treated with DNase I (Qiagen) to remove any contaminating genomic DNA. The amount of total RNA was quantified with the Hitachi DNA spectrophotometer and the quality was analyzed with 1% formaldehyde-agarose gel. cDNA was synthesized with SuperScript cDNA synthesis III kit (Invitrogen) in accordance with the manufacturer's instructions.
Quantitative RT-PCR was used to detect IL-1β and TNF-α transcripts using β2M as an endogenous control in the cells. PCR amplification was performed with 2×QantiTect SYBR Green PCR Master mix (Qiagen) with each validated primer (Qiagen) according to the manufacturer's protocol. Levels of mRNA were measured by a Chromo 4 (MJ Research). For relative quantification, the expression of each gene was normalized to the expression of β2M in the cells relative to a calibrator. The amount of target was represented by 2-ΔΔCt.
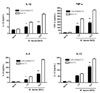
Macrophages have phagocytic properties that may contribute to the presentation of microbial structure to intracellular sensors. To examine the impact of phagocytosis on cytokine production, RAW264.7 cells were pre-treated with cytochalasin D before stimulation with M. leprae. We assumed that the inhibitory effect of cytochalasin D on phagocytosis may affect the cytokine production by macrophage infected with M. leprae. As we expected, treatment with cytochalasin D decreased the production of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, compared to non-treated group (Fig. 1). However, cytochalasin D had no effect on cytokine production after 24 h stimulation with M. leprae (data not shown). We concluded that the impact of phagocytosis is dependent on the early entry to the cells.
As bacterial entry into cells was important in cytokine production by macrophages, we were interested in the role of cytosolic receptor, NODs, for recognizing the molecular pattern from pathogen phagocytosed into cell. Therefore, the contribution of the NOD proteins to macrophage activation by M. leprae was examined by measuring the activation of NF-κB and the expression of proinflammatory cytokines. To investigate the role of NOD1 and NOD2 in the response of the host cell against M. leprae infection, HEK cells were transfected with NOD1 or NOD2 expression plasmid, and then the ability of M. leprae to activate these pathogen recognition receptors was assessed by monitoring the level of NOD-dependent expression of the pro-inflammatory cytokines, IL-1β and TNF-α. Whereas M. leprae increased the activation of NF-κB in the cell transfected with NOD1 or NOD2 expression plasmid, treatment with cytochalasin D decreased the NF-κB activation in the cells (Fig. 2A). We also observed that NODs-transfected cells induced significantly IL-1β (Fig.2 B) and TNF-α (Fig. 2C) expression to M. leprae in mRNA level. Inhibitory effect of cytochalasin D on cytokine expression in macrophages was also no longer observed after 24 h stimulation (data not shown).
As Ferwerda et al. (12) reported that the host response to M. tuberculosis was NOD2-dependent, we assessed whether the immune response to M. leprae was dependent on NOD1 or NOD2 (Fig. 2). There is no difference between NOD1 and NOD2 in response of cells to M. leprae infection. However, whereas there was minimal response to M. leprae in NOD1-transfected cells, a significantly higher response was in NOD2-transfected cells. Our future study will investigate the role of NOD2 in host infected with M. leprae using siRNA and knock-out mice. RICK (also called RIP2) is a caspase-recruitment domain (CARD)-containing kinase that is a critical downstream mediator of NOD1 and NOD2 signaling and plays a role in host defense against intracellular bacteria (14-16). Therefore, the study about the relationship between NOD2 and RICK expression in host response against M. leprae infection will be also added in our future study.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download