Abstract
Since CKD-712 has been developed as an anti-inflammatory agent, we examined the effect of CKD-712 during TLR4 signaling. Using HEK293 cells expressing TLR4, CKD-712 was pre-treated 1 hr before LPS stimulation. Activation of NF-κB was assessed by promoter assay. The activation of ERK, JNK, p38, IRF3 and Akt was measured by western blotting. CKD-712 inhibited the NF-κB signaling triggered by LPS. The activation of ERK, JNK, p38 or IRF3 was not inhibited by CKD-712. On the contrary the activation of these molecules was augmented slightly. The activation of Akt with stimulation of LPS was also enhanced with CKD-712 pre-treatment at lower concentration, but was inhibited at higher concentration. We suggest that during TLR4 signaling CKD-712 inhibits NF-κB activation. However, CKD-712 augmented the activation of Akt as well as Map kinases. Therefore, we suggest that CKD-712 might have a role as an immunomodulator.
Immunomodulation has been an interesting phenomenon among immunologists since immunopotentiation could enhance immunity against infection or tumors, whereas immunosupression or anti-inflammatory manipulation can be used to control autoimmunity or diseases caused by aberrant or hyper-reactive immune responses. Sepsis is caused by hyper-responsiveness of the immune system, in which the mortality rate is as high as 50% (1). In septic conditions, large amount of pro-inflammatory cytokines is produced rapidly. Therefore, the early innate response of the immune system to pathogens plays a critical role in septic conditions. Toll-like receptors (TLRs) are important pattern recognition receptors (PRRs) that participate in the innate immune response. TLR4, in conjunction with CD14, acts as the receptor for lipopolysaccharide (LPS), a component of Gram-negative bacteria cell walls (2). TLR2 recognizes peptidoglycan and lipoteichoic acid (LTA) in Gram-positive bacteria cell walls (3). Activation of TLR2 or TLR4 through binding of their ligands leads to NF-κB activation and transcription of many genes, including typical pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6 as well as several chemokines that recruit leukocytes into the site of inflammation (4,5).
CKD-712 has been developed as an anti-inflammatory agent targeting sepsis. CKD-712 is a higenamine derivative (1-alpha-naphthylmethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline), also referred to as YS 49. It has been shown that CKD-712 reduced NO production in macrophage cells and increased survival rates in mice after LPS treatment (6,7). Furthermore inhibition of the Janus activated kinase (JAK)-2/signal transducer and activators of transcription (STAT)-1 pathway by CKD-712 has been reported to decrease levels of iNOS, cyclooxygenase-2, and hemeoxygenase-1 in macrophage cells (8). More recently, CKD-712 was found to suppress the secretion of high-mobility group box 1 (HMGB-1) via inhibition of phosphatidylinositol 3 (PI3K)-protein kinase C (PKC) signaling after LTA or LPS stimulation in macrophage cells (9).
In this study we attempted to determine which signaling pathway is affected by CKD-712 after TLR4 stimulation, since TLR4 is the major PRRs responsible in development of sepsis.
HEK293-TLR4 cells (kindly provided by Professor Douglas T. Golenbock, Massachusetts University, USA) were cultured in 10% fetal bovine serum-RPMI1640 medium. HEK293-TLR4 cells express MD2 and CD14 as well. CKD-712 was kindly provided by ChongKunDang Pharm, Cheonan. LPS was purchased from Sigma-Aldrich, St. Louis, MI, USA. To assess cell viability after treatment of CKD-712, cells were reacted with 5 mg/ml of tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, MTT) (Sigma-Aldrich). After 3 hrs of incubation (37℃, 5% CO2) the medium containing MTT was removed and 200µl of DMSO was added into the well and the absorbance was measured at 540 nm. For the luciferase assay. 2×105 cells/well were seeded in 12-well plates and cultured for 2 days. NF-κB promoter and pCMV β-gal vector were co-transfected overnight, and CKD-712 was pre-treated before LPS treatment. LPS was treated for 6 hrs. Luciferase activity was measured by a Luciferase Reporter Assay System (Promega, Madison, WI, USA) and β-galactosidase activity was measured with O-nitrophenyl-β-D-galactopyranoside (Sigma-Aldrich), as the substrate. Luciferase activity was normalized for transfection efficiency with the β-galactosidase activity. Western blotting was done using anti-TLR4, anti-ERK, anti-p38, anti-JNK, anti-IRF3 or anti-Akt antibodies as well as control antibodies. All of the antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA).
First, we determined the optimal concentration of CKD-712 in HEK293-TLR4 cells. When treated with CKD-712 for 24 hrs the cell viability was 50% at 100µM and 40% at 200µM (Fig. 1A). Next, HEK293-TLR4 cells were treated with LPS and NF-κB activation was measured. CKD-712 was pre-treated 1 hr before stimulation of LPS. We found that 50µM of CKD-712 inhibited NF-κB activation in TLR4 signaling. When treated with 50µM of CKD-712, NF-κB activation decreased to less than 100,000 RIU (25 ng/ml of LPS) and 200,000 RIU (50 ng/ml of LPS) when compared to 200,000 RIU and 500,000 RIU, respectively in control groups (Fig. 1B).
Next, we investigated whether treatment with CKD-712 modulates MAP kinaseactivation. After stimulation with LPS, CKD-712 did not inhibit activation of ERK, JNK or p38 kinase in HEK293-TLR4 cells (Fig. 2). Rather, pre-treatment with CKD-712 delayed activation of these kinases. As shown in Fig. 3, MAP kinases were phosphorylated 15~30 min following stimulation with LPS, after which activation subsided in 60 min. However, CKD-712 treatment extended the activation of ERK and JNK, which were found to be activated 60 min after TLR4 stimulation (Fig. 2). Phosphorylation of IRF3 was not inhibited and showed a sustained activation like the MAP kinases (Fig. 2). Interestingly, the activation of Akt was augmented slightly with pre-treatment of CKD-712 (50µM) after LPS stimulation (Fig. 3, left). But higher concentration of CKD-712 (100µM) inhibited the activation of Akt completely (Fig. 3, right).
With the brief data presented here, we suggest that CKD-712 activated Akt and MAP kinases after TLR4 stimulation at 50µM. Emerging evidence has shown the existence of a close relation between TLRs and the PI3K/Akt pathway. One of the early evidences demonstraetd that PI3-kinase is involved in TLR4-mediated cytokine expression in mouse macrophages (10). Even in non-immune cells, such as, in foam cells, binding of LPS and TLR4 increases Nox1 expression through the phospholipase A2 β-Akt signaling pathway (11). Another paper reporting that HMGB-1 induced migration of vascular smooth muscle cells in the TLR4-dependent PI3K/Akt pathway also suggests a strong involvement of PI3K/Akt in the TLR4 signaling pathway (12).
But the PI3K-Akt-mTOR-p70 S6k pathway is also known to be a negative regulator of MyD88-dependent intracellular signals after TLR stimulation. TLR4-mediated signaling leads to rapid activation of PI3K and phosphorylation of Akt, a downstream target of PI3K in mouse macrophages, in which the sustained interaction of MyD88/PI3K with the TLR4 intracellular "signaling platform" negatively regulates further MyD88 dependent signaling (13). The administration of the TLR2 ligand, Pam3CSK4, was found to significantly increase the levels of phosphorylation of Akt, which lead to significant protection in a cerebral injury model, suggesting that the PI3 kinase-Akt pathway is involved with TLR2 signaling as well as TLR4 signaling (14). More recently the association of endogenous TBK1 and Akt was observed in macrophages when stimulated with poly (I:C) and LPS, showing that Akt activation mediated by TBK1 contributes to TLR3 and TLR4-mediated immune responses (15).
The effect of CKD-712 on phosphorylation of Akt depends on the cell types. In immune cells, CKD-712 inhibits Akt activation in macrophages (9) and human endothelial cells (15). But in rat heart muscle cells, CKD-712 enhances Akt activation to prevent myocardical ischemia and further inflammation (16). Our data show that in HEK293 cells, CKD-712 at low concentration augments the activation of Akt, whereas high concentration of CKD-712 has an inhibiting effect. Therefore pharmaceutical agents regulating the PI3-Akt pathway may be used as immunomodulators in the innate immune response. Although the mechanism of the augmented activation of Akt by CKD-712 and the inhibition of NF-κB activation have yet to be clarified, CKD-712 still has a potential as an immunomodulator.
Figures and Tables
 | Figure 1Effect of CKD-712 on TLR4 signaling. (A) To assess CKD-712 toxicity HEK293-TLR4 cells (2×105) were cultured with CKD-712 for 24 hrs and cell viability was determined by MTT assay. (B) To evaluate the effect of CKD-712 on NF-κB activation 2×105 HEK-TLR4 cells/well were seeded in 12-well plates and were cultured for 2 days. Then NF-κB promoter and pCMV β-gal vector were co-transfeceted overnight and CKD-712 (50µM) was pre-treated before LPS treatment. LPS was treated for 6 hrs. After measuring luciferase activity and β-galactosidase activity the luciferase activity was normalized for transfection efficiency with the β-galactosidase activity. |
 | Figure 2Effect of CKD-712 on activation of MAP kinases and IRF3 in TLR4 signaling. HEK293-TLR4 cells were stimulated with LPS (50 ng/ml). Western blot was performed anti-ERK, anti-JNK, anti-p38 or anti-IRF3 antibodies. CKD-712 (50µM) was pre-treated 1 hr before LPS stimulation. Three independent experiments were performed and the representative data are shown. |
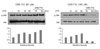 | Figure 3Effect of CKD-712 on Akt activation in TLR4 signaling. HEK293-TLR4 cells were stimulated with LPS (50 ng/mL) and western blot was performed with anti-Akt antibodies. CKD-712 (50 or 100µM) was pretreated 1 hr before LPS stimulation. Two independent experiments were performed and the representative data are shown. The density was measures by densitometry and relative ratio of p-Akt/Akt in study groups was calculated based on the p-Akt/Akt of control group as 1.0. |
ACKNOWLEDGEMENTS
This work is supported by the grant provided from CKD Research Institute. The authors would like to thank Jong Soo Kim for his assistance in editing of this manuscript.
References
1. Clermont G, Angus DC, Kalassian KG, Linde-Zwirble WT, Ramakrishnan N, Linden PK, Pinsky MR. Reassessing the value of short-term mortality in sepsis: comparing conventional approaches to modeling. Crit Care Med. 2003. 31:2627–2633.

2. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998. 282:2085–2088.

3. Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999. 274:17406–17409.

4. Flo TH, Halaas O, Lien E, Ryan L, Teti G, Golenbock DT, Sundan A, Espevik T. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000. 164:2064–2069.

5. Tamandl D, Bahrami M, Wessner B, Weigel G, Ploder M, Fürst W, Roth E, Boltz-Nitulescu G, Spittler A. Modulation of toll-like receptor 4 expression on human monocytes by tumor necrosis factor and interleukin-6: tumor necrosis factor evokes lipopolysaccharide hyporesponsiveness, whereas interleukin-6 enhances lipopolysaccharide activity. Shock. 2003. 20:224–229.

6. Kang YJ, Koo EB, Lee YS, Yun-Choi HS, Chang KC. Prevention of the expression of inducible nitric oxide synthase by a novel positive inotropic agent, YS 49, in rat vascular smooth muscle and RAW 264.7 macrophages. Br J Pharmacol. 1999. 128:357–364.

7. Kang YJ, Seo SJ, Yun-Choi HS, Lee DH, Kim YM, Chang KC. A synthetic isoquinoline alkaloid, 1-(beta-naphthylmethyl)-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (YS 51), reduces inducible nitric oxide synthase expression and improves survival in a rodent model of endotoxic shock. J Pharmacol Exp Ther. 2002. 301:561–567.

8. Tsoyi K, Kim HJ, Shin JS, Kim DH, Cho HJ, Lee SS, Ahn SK, Yun-Choi HS, Lee JH, Seo HG, Chang KC. HO-1 and JAK-2/STAT-1 signals are involved in preferential inhibition of iNOS over COX-2 gene expression by newly synthesized tetrahydroisoquinoline alkaloid, CKD712, in cells activated with lipopolysacchride. Cell Signal. 2008. 20:1839–1847.

9. Oh YJ, Youn JH, Min HJ, Kim DH, Lee SS, Choi IH, Shin JS. CKD712, (S)-1-(α-naphthylmethyl)-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline, inhibits the lipopolysaccharide-stimulated secretion of HMGB1 by inhibiting PI3K and classical protein kinase C. Int Immunopharmacol. 2011. 11:1160–1165.

10. Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003. 33:597–605.

11. Lee SH, Park DW, Park SC, Park YK, Hong SY, Kim JR, Lee CH, Baek SH. Calcium-independent phospholipase A2beta-Akt signaling is involved in lipopolysaccharide-induced NADPH oxidase 1 expression and foam cell formation. J Immunol. 2009. 183:7497–7504.

12. Yang J, Chen L, Yang J, Ding J, Rong H, Dong W, Li X. High mobility group box-1 induces migration of vascular smooth muscle cells via TLR4-dependent PI3K/Akt pathway activation. Mol Biol Rep. 2011. [Epub ahead of print].

13. Lu C, Liu L, Chen Y, Ha T, Kelley J, Schweitzer J, Kalbfleisch JH, Kao RL, Williams DL, Li C. TLR2 ligand induces protection against cerebral ischemia/reperfusion injury via activation of phosphoinositide 3-kinase/Akt signaling. J Immunol. 2011. 187:1458–1466.

14. Joung SM, Park ZY, Rani S, Takeuchi O, Akira S, Lee JY. Akt contributes to activation of the TRIF-dependent signaling pathways of TLRs by interacting with TANK-binding kinase 1. J Immunol. 2011. 186:499–507.

15. Tsoyi K, Kim WS, Kim YM, Kim HJ, Seo HG, Lee JH, Yun-Choi HS, Chang KC. Upregulation of PTEN by CKD712, a synthetic tetrahydroisoquinoline alkaloid, selectively inhibits lipopolysaccharide-induced VCAM-1 but not ICAM-1 expression in human endothelial cells. Atherosclerosis. 2009. 207:412–419.

16. Jin YC, Lee YS, Kim YM, Seo HG, Lee JH, Kim HJ, Yun-Choi HS, Chang KC. (S)-1-(alpha-naphthylmethyl)-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (CKD712) reduces rat myocardial apoptosis against ischemia and reperfusion injury by activation of phosphatidylinositol 3-kinase/Akt signaling and anti-inflammatory action in vivo. J Pharmacol Exp Ther. 2009. 330:440–448.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download