Abstract
Dextran sodium sulfate (DSS) is a widely used chemical model for inflammatory bowel disease (IBD). It is thought that imbalances in the T helper (Th) cell subsets contribute to IBD. Recent studies suggest that the acute DSS-colitis model is polarized toward a Th1/Th17 profile based on RT-PCR analysis of colonic tissues. In the current study we determined whether colonic Th cells from DSS-colitis mice were skewed toward the Th17 profile. Mice were treated with 5% DSS for 7 days and colonic T cells isolated and examined for production of IFN-γ (Th1 cell), IL-4 (Th2 cell) and IL-17 (Th17 cell) by intracellular flow cytometry. We found that the percentage of colonic Th17 cells were similar to non-treated controls but the percentage of Th1 cells were elevated in DSS-colitis mice. These results suggest that in the acute DSS-colitis model the colonic Th cells exhibit a Th1 profile and not a Th17 profile.
Inflammatory bowel disease (IBD) is defined as a chronic inflammatory disorder of the gastrointestinal tract and is classified as Crohn's disease or ulcerative colitis based on distinct clinical, endoscopic and histopathological features. Multiple studies suggest that microbial, environmental and genetic factors contribute to the pathogenesis of this disorder [reviewed in (1,2)]. These factors ultimately culminate in an inappropriate adaptive immune response which perpetuates the chronic intestinal inflammation. Much of our mechanistic understanding of IBD pathogenesis has been extrapolated from studies of animal models (3). Among these animal model systems, the oral uptake of dextran sodium sulfate (DSS) by mice induces a phenotype similar to ulcerative colitis and is widely used by researchers to study IBD (4).
DSS damages the colonic epithelial barrier which then exposures the underlying tissues to bacterial flora and results in subsequent inflammation and induction of Th cell responses. The murine DSS-induced colitis model recapitulates many aspects of human IBD such as cytokine dysregulation which is a key feature of IBD pathogenesis (5). This dysregulation is thought to be due to the imbalance of T helper (Th) cell subsets such as T helper 1 (Th1) and T helper 2 (Th2) cells. Crohn's disease is characterized by a Th1 cytokine profile mediated by the cytokines IFN-γ, IL-12 and TNF-α. In contrast, ulcerative colitis is characterized by a Th2 cytokine profile mediated by the cytokines IL-4 and IL-5. However, this simplified classification has been challenged by studies suggesting that Crohn's disease is characterized by the production of IL-17 (6,7). In an acute model of DSS-colitis, Alex et al. showed that mice give DSS for 7 days exhibited an increase in IL-12 and IL-17 suggesting an induction of a Th1/Th17 profile (7). However, in this study protein and mRNA samples from whole colonic tissues and serum were analyzed and not Th cells. Therefore, it is unclear if indeed the Th cells were also polarized toward the Th1/Th17 subset. In this current report, we examined the percentages of Th1/Th2/Th17 cells in the colonic tissues of DSS-treated mice to determine whether the Th cells were polarized to the Th1 and/or Th17 subset.
Six-week-old female C57Bl/6 mice (ORIENT BIO, Korea) powere given 5% (w/v) DSS (MW 30,000~45,000, MP Bioche-pulation. micals, Solon, OH, USA) in the drinking water and fed ad libitum for 7 days. Control mice received water without DSS. This study was approved by the Institutional Review Board of Yonsei University at Wonju, South Korea. We isolated the colonic lymphocytes and conducted intracellular staining by flow cytometry as previously described (8). In brief, pooled large intestines (3 mice per group) were minced and enzymatically digested with Liberase™ (Roche, USA) and DNase I (Roche). The lymphocytes were isolated by using a Percoll gradient. Cells were stimulated for 4 hr in vitro in the presence of PMA (30 nM) (Promega, USA), ionomycin (1µM) (Sigma, USA) and GolgiPlug™ (BD Biosciences, USA). Then the cells were washed, fixed and stained for cell surface markers and intracellular cytokines. Cells were analyzed using FACSCalibur (BD Biosciences).
Mice administered DSS developed fulminant colitis as determined by hematoxylin and eosin (H&E) staining of the colon (Fig. 1). In addition, the DSS-treated mice exhibited rectal bleeding, decrease in body weight and shortening of the colon length which are all characteristic features of DSS-colitis (data not shown) (4). We analyzed the Th cell population (i.e., CD3+4+ T cells) for production of the intracellular cytokines IFN-γ, IL-4 and IL-17 and found that compared to the control mice Th cells from the DSS-treated mice did not show an increase in IL-17 producing CD3+4+ T cells (DSS 6.8% versus control 8.3%) (Fig. 2). Similarly, the percentages of IL-4 producing CD3+4+ T cells were comparable in both groups of mice (DSS 2.2% versus control 2.6%). In contrast, the percentages of IFN-γ producing CD3+4+ T cells were increased two-fold in the DSS-treated group compared to the control group (DSS 12.1% versus control 5.6%). These results indicate that in the acute DSS-colitis model the Th cells exhibit a predominantly Th1 profile.
Ostensibly these results appear in conflict with several recent studies that were published during the preparation of this manuscript (9-12). The results from these studies suggest that the DSS colitis model is Th1/Th17 polarizing. The aforementioned studies differ from our study in that the mouse strains used, DSS dose and DSS administration times are different. Studies have demonstrated that the DSS dose affects the extent of colonic inflammation which may also lead to different Th differentiation programs (13). The results from this current study indicate that in the acute DSS-colitis model the Th cell cytokine profile is strictly Th1 polarizing. Therefore, we suggest that the IL-17 production in the DSS model is due to other cell types. Several studies have indicated IL-17 can be produced by CD8+ T cells (14), NKT cells (15,16), neutrophils (17) and paneth cells (18). Recent studies have shown that the colonic lamina propria γ/δ T cells can also be a source of IL-17 producing cells (8,19). We did not find an increase in CD3+4- IL-17 producing cells in the DSS treated mice suggesting that γ/δ T cells are not a major contributor of IL-17 in this model (data not shown).
In Crohn's disease, the cytokine makeup is considered to be skewed toward the Th1/Th17 profile whereas in ulcerative colitis Th2 profile dominates. Therefore, our data suggests that although the murine DSS model histopathologically mimics ulcerative colitis the Th response is not Th2. Additionally, the Th1/Th17 response observed in human Crohn's disease was also not mirrored in the murine DSS-colitis model. The results from this current study raise a cautionary note when extrapolating results from murine DSS studies to human IBD.
Our flow cytometric results suggest that the Th response is skewed toward the Th1 profile in our DSS model. However, supplemental experiments examining mRNA levels of the cytokine genes IFN-γ, IL-4 and IL-17 by real-time PCR would provide a further validation for our conclusion. Similarly, examining expression levels of the Th cell promoting transcription factors T-bet (Th1), GATA-3 (Th2) and ROR-γ (Th17) from lamina propria CD3+CD4+ sorted cells should provide a more comprehensive and definitive answer. In conclusion, we provide evidence suggesting that in the murine model of acute DSS-colitis the colonic T cells are polarized to a Th1 profile and not a Th17 profile.
Figures and Tables
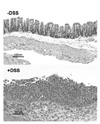 | Figure 1H&E staining of colons from control mice and DSS-treated mice. Mice were administered 5% DSS in the drinking water for 7 days. |
 | Figure 2Intracellular cytokine flow cytometric analysis of colonic CD3+4+T cells from the large intestines of DSS-treated mice and control mice. After one week of DSS treatment, isolated lymphocytes were treated with PMA, ionomycin, GolgiPlug™ for 4 hours and stained with antibodies against cell surface markers and cytokines. Dot plots were derived from cells gated on the CD3+4+ T cell population. Data are representative of three independent experiments. The numbers inside each quadrant indicate the percentage of the cell population.
|
ACKNOWLEDGEMENTS
This work was supported in part by the New Investigator Fund from Yonsei University at Wonju (#2011-5-5018).
References
1. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007. 448:427–434.

2. Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007. 117:514–521.

4. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990. 98:694–702.

5. Pizarro TT, Cominelli F. Cytokine therapy for Crohn's disease: advances in translational research. Annu Rev Med. 2007. 58:433–444.

6. Fuss IJ, Becker C, Yang Z, Groden C, Hornung RL, Heller F, Neurath MF, Strober W, Mannon PJ. Both IL-12p70 and IL-23 are synthesized during active Crohn's disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm Bowel Dis. 2006. 12:9–15.

7. Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009. 15:341–352.

8. Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009. 15:1016–1022.

9. Brown JB, Cheresh P, Zhang Z, Ryu H, Managlia E, Barrett TA. P-selectin glycoprotein ligand-1 is needed for sequential recruitment of T-helper 1 (Th1) and local generation of Th17 T cells in dextran sodium sulfate (DSS) colitis. Inflamm Bowel Dis. 2011. [Epub ahead of print].

10. Sanada Y, Mizushima T, Kai Y, Nishimura J, Hagiya H, Kurata H, Mizuno H, Uejima E, Ito T. Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLoS One. 2011. 6:e23933.

11. Nuñez-Andrade N, Lamana A, Sancho D, Gisbert JP, Gonzalez-Amaro R, Sanchez-Madrid F, Urzainqui A. P-selectin glycoprotein ligand-1 modulates immune inflammatory responses in the enteric lamina propria. J Pathol. 2011. 224:212–221.

12. Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Iwakura Y, Okanoue T, Yoshikawa T, Kataoka K, Mazda O. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008. 377:12–16.

13. Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Büchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000. 62:240–248.

14. Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, Laurence A, Michalowska A, Mamura M, Lonning S, Berzofsky JA, Wakefield LM. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008. 68:3915–3923.

15. Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008. 205:385–393.

16. Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008. 180:5167–5171.

17. Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003. 170:2106–2112.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download