Abstract
Background
Endogenous uveitis is a chronic inflammatory eye disease of human, which frequently leads to blindness. Experimental autoimmune uveoretinitis (EAU) is an animal disease model of human endogenous uveitis and can be induced in susceptible animals by immunization with retinal antigens. EAU resembles the key immunological characteristics of human disease in that both are CD4+ T-cell mediated diseases. Dendritic cells (DCs) are specialized antigen-presenting cells that are uniquely capable of activating naïve T cells. Regulation of immune responses through modulation of DCs has thus been tried extensively. Recently our group reported that donor strain-derived immature DC pretreatment successfully controlled the adverse immune response during allogeneic transplantation.
Methods
EAU was induced by immunization with human interphotoreceptor retinoid-binding protein (IRBP) peptide1-20. Dendritic cells were differentiated from bone marrow in the presence of recombinant GM-CSF.
Endogenous uveitis is a chronic inflammatory eye disease, leading to blindness not infrequently (1). Even though, uveitis often appears in association with systemic diseases such as Vogt-Koyanagi-Harada disease or Behcet's disease, the majority of uveitis is still of an idiopathic origin (2). Experimental autoimmune uveoretinitis (EAU) is a disease model of human endogenous uveitis and can be induced in susceptible animals by immunization with retinal antigens (3). Uveitogenic antigens which can induce EAU are retinal soluble antigen, interphotoreceptor retinoid-binding protein (IRBP), rhodopsin, opsin, recoverin, and phosducin (4). EAU is a CD4+ T cell-mediated disease. Uveitogenic effector CD4+ T cells infiltrate into the eyes and are responsible for the pathogenesis (5).
Dendritic cells (DCs) are specialized antigen-presenting cells that are uniquely capable of activating naive T cells (6). When pathogens invade the tissue, tissue-resident DCs endocytose antigens and process them for presentation on the MHC molecules. With appropriate inflammatory signals provoked by pathogens, DCs upregulated the expression of surface MHC molecules containing antigenic peptides and co-stimulatory molecules on their surface (7). High level surface expression of MHC class II and co-stimulatory molecules are whole-mark of activated dendritic cells and are prerequisite for effective activation of naive CD4+ T cells (8). In contrast to activated DCs, immature DCs with low levels of MHC and co-stimulatory molecules have been implicated in the regulation of immune responses through diverse effector mechanisms (9). Particularly, immature DCs are able to induce a state of hyporesponsiveness in T cells, the phenomenon has been used for the control or suppression of the immune response (10). Therefore, numerous attempts have been made to modify DCs to retain their immature phenotype for the induction of antigen-specific tolerance (11,12). Recently our group reported that donor strain-derived immature DC pretreatment successfully controlled the immune response during allogeneic transplantation (13).
In this study, we used paraformaldehyde-fixed bone marrow (BM)-derived DCs to maintain them in an immature state. Pretreatment with fixed immature DCs, but not fixed mature DCs, ameliorated the disease progression of experimental uveoretinitis by inhibiting uveitogenic CD4+ T cell activation and differentiation.
C57BL/6 (B6) female mice (Jackson, Bar Harbor, MA), 8-10 weeks of age, were immunized s.c in the both footpads and tail-base with 250µg of human interphotoreceptor retinoidbinding protein (IRBP) peptide1-20 (GPTHLFQPSLVLDMAKVLLD) (Peptron, Daejeon, Korea) in 100µl of emulsion in complete Freund's adjuvant (Sigma-Aldrich, St. Louis, MO) supplemented with Mycobacterium tuberculosis (strain H37 Ra; Difco, Detroit, MI) to 1.5 mg/ml (14). Mice were received 1.0µg of pertussis toxin (Sigma-Aldrich), intraperitoneally at the time of the immunization. All mice were bred and maintained in specific pathogen-free conditions at the animal facility of Seoul National University College of Medicine. All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC, SNU 050502003) at Seoul National University.
Eyes were removed from mice sacrificed 21 days after the immunization with human IRBP peptide, and then fixed in 4% buffered paraformaldehyde (PFA), embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histopathological analysis. The severity of the disease was determined for each eye and scored on a scale of 0~4 in halfpoint increments according to a semiquantitative system (15).
Bone marrow cells from the both femurs and tibias were obtained from 6~8-week-old mice. Cells were passed through a nylon mesh and red blood cells were lysed. Following washing, granulocytes, B cells and red blood cell precursors were removed by magnetic beads (Miltenyi Biotec, Auburn, CA) using a monoclonal antibody cocktail of anti-GR1, anti-TER119, and anti-B220. 5×105 cells/ml were plated in 24-well plates in 1 ml of medium supplemented with 20 ng/ml recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF; BioSource, Camarillo, CA). The cultures were fed every 2 days by gently swirling the plates, aspirating 50% of the medium, and adding fresh medium containing rGM-CSF. Dendritic cells (DCs) were harvested 6 days after bone-marrow culture by removing Gr1+ granulocytes from cultures using magnetic beads. To induce maturation of DCs, 1µg/ml lipopolysaccharide (Salmonella abortus equi; Sigma-Aldrich) was added to the cell culture for 18 hrs before harvest. To render DCs maturation-resistant, they were fixed by resuspension in 2% (weight/volume) paraformaldehyde for 10 min, then washed three times. Fixed immature or fixed mature cells were used for the following experiments.
CD4+ T cells from draining inguinal and popliteal lymph nodes of mice were purified using magnetic beads on day 7 after immunization with human IRBP peptide, then co-cultured (2×105 cells/well) with γ-irradiated, syngeneic splenocytes (4×105 cells/well) with or without 30µM of IRBP peptide in round-bottom 96 well plate. Culture was incubated for 72 hrs at 37℃ in a 5% CO2 atmosphere, pulsed [3H]-thymidine (1µCi/well) during the last 12 hrs, then incorporated radioactivity was counted.
In vitro generated DCs from bone marrow of GFP transgenic mice (Jackson, Bar Harbor, MA) were fixed in 2% PFA and injected intravenously with 5×106 cells into B6 mice. On 14 days after injection with DCs, spleens and inguinal lymph nodes were frozen in OCT compound, sectioned (4µm), and examined under fluorescence microscopy.
After restimulation, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100. Cells were stained with anti-CD4, anti-CD62L, and anti-IFN-γ (all from BD-Pharmingen, San Diego, CA). Flow cytometric analysis was conducted using a FACSCalibur (BD Bioscience, San Jose, CA).
Female C57BL/6 (B6) mice were immunized with an uveitogenic human IRBP 1~20 peptide. Two days before immunization, mice were intravenously injected with 5×106 BM-derived fixed immature DCs (iBMDC), fixed mature DCs (mBMDC), or media. B6 mice pretreated with mBMDC developed more severe uveoretinitis compared with the moderate disease in media-pretreated mice, whereas mice pretreated with iBMDC revealed improved disease phenotype compared with media-pretreated control. Extensive tissue damage, including retinal folding, heavy inflammatory cell infiltration into the vitreous humor, and choroidal granuloma formation were noted in the eyes of mBMDC-pretreated mice (Fig. 1A). In contrast to this, mice pretreated with iBMDC exhibited very mild inflammatory cell infiltration and local retinal destruction (Fig. 1A). The median disease scores were 1.8 (mBMDC-pretreated), 0.6 (iBMDC-pretreated), and 1.4 (media-pretreated) (Fig. 1B). IRBP-specific CD4+ T lymphocyte proliferation prepared from the draining lymph nodes was greatly reduced in iBMDC-pretreated mice compared to the media-pretreated control (Fig. 1C).
To evaluate the initial uveitogenic CD4+ T cell responses following immunization in the mBMDC or iBMDC-pretreated mice, lymphocytes prepared from draining lymph nodes were analyzed on the 7th day after immunization for the activation marker. CD62L-negative activated T cells among CD4+ T cells were increased following immunization with IRBP peptide while iBMDC pretreatment decreased the percentage of activated CD4+ T cells compared to the media-pretreated control (Fig. 2).
Uveitogenic CD4+ T cell differentiation in the draining lymph nodes were also analyzed on the 7th day after immunization. IFN-γ-expressing CD4+ T cells among uveitogenic T cells were decreased in the iBMDC-pretreated mice and increased in the mBMDC-pretreated mice compared with media-pretreated control mice (Fig. 3).
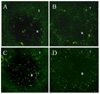
To evaluate the site of immunoregulation on the activation and differentiation of uveitogenic CD4+ T cells implemented by pretreated iBMDC, B6 mice were received iBMDC and mBMDC prepared from syngeneic GFP transgenic mice. On 14 days after injection with DCs, spleens and inguinal lymph nodes were examined under fluorescence microscopy. Both the fixed immature DCs and fixed mature DCs migrated into the T-cell zones of secondary lymphoid organs such as spleen and inguinal lymph nodes and stayed there at least for 14 days following injection (Fig. 4).
In this study, we demonstrated that pretreatment with fixed immature bone marrow-derived dendritic cells (iBMDC) ameliorated experimental autoimmune uveoretinitis (EAU) by inhibiting uveitogenic CD4+ T cell activation and differentiation. Pretreated fixed immature dendritic cells migrated into the T-cell zone of the secondary lymphoid organs and affected the uveitogenic CD4+ T cell immune responses.
EAU is an animal disease model of human endogenous uveitis and resembles the key immunological characteristics of human disease in that both are CD4+ T-cell mediated diseases (5). In EAU pathogenesis, Th1 immune responses have been suggested to be essential factors. Susceptibility to disease paralleled Th1 responsiveness among the different mouse or rat strains (16). In this study we found that pretreated iBMDC effectively inhibited uveitogenic Th1 differentiation in the draining lymph nodes. As the transferred fixed dendritic cells migrated and survived in the secondary lymphoid organs for more than 2 weeks, we assumed that iBMDC directly inhibited initial uveitogenic CD4+ T cell activation and subsequent differentiation in the T-cell zone of these organs. Recently, Th17 cells have also been implicated in the disease progression of autoimmune eye diseases of human and animal models, including uveitis. Th17 cells among peripheral blood lymphocytes were increased in active uveitis patients and treatment with blocking anti-IL-17 antibody mitigated EAU in animal models (17). IFN-γ and IL-17 were suggested to have distinct pathogenic roles in different animal models of autoimmune uveoretinitis uveiretinitis (18,19). Thus, evaluation of the effect of iBMDC on the Th17 differentiation of uveitogenic CD4+ T cells would be very important in the following study.
As the immature DCs are important in maintaining self tolerance, many researchers have tried to regulate autoimmune response using immature DCs (8). However, previous attempts have not obtained consistent results, possibly because the immaturity of transferred DCs have not sustained in vivo and they mature spontaneously following injection and so can activate the immune responses (20,21). To overcome this phenomenon, we treated DCs with paraformaldehyde to render them unable to change their phenotypes. We used 2% paraformaldehyde as the fixative agent in our experiments because we successfully controlled allogeneic T cell response by pretreating iBMDC prepared according to this protocol (13). Pretreatment of iBMDC also ameliorated experimental autoimmune uveoretinitis in this study, while mBMDC pretreatment aggravated the disease. One caveat for this protocol of DC preparation was that fixation might have destroyed the ability of DCs to function as antigen presenting cells and/or affected the survival of DCs in the recipient. Paraformaldehyde fixation, however, did not eliminate the potential of DCs in supporting allogeneic T-cell proliferation (13). Also as both the fixed immature DCs and fixed mature DCs survived over 2 weeks in the secondary lymphoid organs of the recipients, the concern of second possibility was ruled out. Thus, application of iBMDC prepared according to the protocol of this study would provide an important treatment modality for the autoimmune diseases and transplantation rejection.
Figures and Tables
Figure 1
Mature-resistant DC ameliorates EAU in mice. (A) Histology of eyes. C57BL/6 (B6) mice were intravenously injected with 5×106 BM-derived fixed immature DCs (iBMDC), fixed mature DCs (mBMDC), or media. Two days after the BMDC transfer, mice were immunized with human IRBP peptide1-20, Both eyes were removed, fixed in 4% paraformaldehyde, and embedded in paraffin. Sections (4µm) were prepared and stained with hematoxylin and eosin (original magnification, ×100). Midsagittal section for each eyes were used. V, vitreous; GL, ganglion layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigmented epithelium (RPE); Ch, choroid. Retina folding and infiltrations (arrows). (B) Histopathological score of EAU. Data are presented as the mean±SEM of n=10 mice for each group from two independent experiments. (C) IRBP-specific proliferation of CD4+ T cells. CD4+ T cells from draining lymph nodes were purified with magnetic beads on the 7th day after immunization, then co-cultured (2×105 cells/well) with irradiated, syngeneic splenocytes (4×105 cells/well) with 30 mM of IRBP peptide. The cultures were incubated for 3 days and pulsed with [3H]-thymidine (1 mCi/well) for the last 12 hrs. Data represent means±SD of two independent determinations with draining lymph nodes from n=3 mice/group.
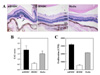
Figure 2
iBMDC-pretreatment reduced the activation of CD4+ T cells. Draining lymph node cells from the mice pretreated with mBMDC, iBMDC, or media and immunized with human IRBP peptide were harvested on the 7th day after immunization. T-cell activation was analyzed using flowcytometer after staining with anti-CD4 and anti-CD62L antibodies. The data are representative of three independent determinations.
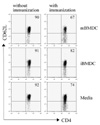
Figure 3
Intracellular cytokine expression of uveitogenic CD4+ T cells. Draining lymph node cells were harvested from the mice received mBMDC, iBMDC, or media on the 7th day after immunization with IRBP peptide and were restimulated in vitro with PMA and ionomycin as described in Materials and Methods. Cells were analyzed using flowcytometer after staining with anti-CD4 and anti-IFN-γ antibodies. The data are representative of two independent determinations.

Figure 4
Both mBMDC and iBMDC migrate into T-cell zone of the spleen and lymph nodes. B6 mice were received mBMDC and iBMDC prepared from GFP transgenic mice. On 14 days after injection with DCs, spleens and inguinal lymph nodes were frozen in OCT compound. Sections (4µm) were examined under fluorescence microscopy (original magnification, ×200). Spleen (A) and inguinal lymph node (B) from the mice received iBMDC. Spleen (C) and inguinal lymph node (D) from the mice received mBMDC. T cell zone (T) and B cell zone (B) were indicated.
ACKNOWLEDGEMENTS
This work was supported by grants from MarineBio Technology Project, Ministry of Land, Transport and Maritime Affairs (D-S.L.).
References
1. Ahn JK, Chung H, Lee DS, Yu YS, Yu HG. CD8brightCD56+ T cells are cytotoxic effectors in patients with active Behcet's uveitis. J Immunol. 2005. 175:6133–6142.

2. Yu HG, Lee DS, Seo JM, Ahn JK, Yu YS, Lee WJ, Chung H. The number of CD8+ T cells and NKT cells increases in the aqueous humor of patients with Behçet's uveitis. Clin Exp Immunol. 2004. 137:437–443.

3. Caspi RR, Chan CC, Wiggert B, Chader GJ. The mouse as a model of experimental autoimmune uveoretinitis (EAU). Curr Eye Res. 1990. 9:Suppl. 169–174.

4. Nussenblatt RB, Caspi RR, Mahdi R, Chan CC, Roberge F, Lider O, Weiner HL. Inhibition of S-antigen induced experimental autoimmune uveoretinitis by oral induction of tolerance with S-antigen. J Immunol. 1990. 144:1689–1695.
5. Sun B, Rizzo LV, Sun SH, Chan CC, Wiggert B, Wilder RL, Caspi RR. Genetic susceptibility to experimental autoimmune uveitis involves more than a predisposition to generate a T helper-1-like or a T helper-2-like response. J Immunol. 1997. 159:1004–1011.
6. Turley SJ. Dendritic cells: inciting and inhibiting autoimmunity. Curr Opin Immunol. 2002. 14:765–770.

7. O'Connell PJ, Li W, Wang Z, Specht SM, Logar AJ, Thomson AW. Immature and mature CD8alpha+ dendritic cells prolong the survival of vascularized heart allografts. J Immunol. 2002. 168:143–154.
8. Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003. 21:685–711.

9. Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003. 18:605–617.

10. Adorini L. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting autoimmune diabetes. Ann N Y Acad Sci. 2003. 987:258–261.

11. Roelen DL, Schuurhuis DH, van den Boogaardt DE, Koekkoek K, van Miert PP, van Schip JJ, Laban S, Rea D, Melief CJ, Offringa R, Ossendorp F, Claas FH. Prolongation of skin graft survival by modulation of the alloimmune response with alternatively activated dendritic cells. Transplantation. 2003. 76:1608–1615.

12. Raimondi G, Thomson AW. Dendritic cells, tolerance and therapy of organ allograft rejection. Contrib Nephrol. 2005. 146:105–120.
13. Kang HG, Lee JE, Yang SH, Lee SH, Gao W, Strom TB, Oh K, Lee DS, Kim YS. Donor-strain-derived immature dendritic cell pre-treatment induced hyporesponsiveness against allogeneic antigens. Immunology. 2010. 129:567–577.

14. Oh K, Byoun OJ, Ham DI, Kim YS, Lee DS. Invariant NKT cells regulate experimental autoimmune uveitis through inhibition of Th17 differentiation. Eur J Immunol. 2011. 41:392–402.

15. Chan CC, Caspi RR, Ni M, Leake WC, Wiggert B, Chader GJ, Nussenblatt RB. Pathology of experimental autoimmune uveoretinitis in mice. J Autoimmun. 1990. 3:247–255.

16. Sun B, Rizzo LV, Sun SH, Chan CC, Wiggert B, Wilder RL, Caspi RR. Genetic susceptibility to experimental autoimmune uveitis involves more than a predisposition to generate a T helper-1-like or a T helper-2-like response. J Immunol. 1997. 159:1004–1011.
17. Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007. 13:711–718.

18. Tang J, Zhu W, Silver PB, Su SB, Chan CC, Caspi RR. Autoimmune uveitis elicited with antigen-pulsed dendritic cells has a distinct clinical signature and is driven by unique effector mechanisms: initial encounter with autoantigen defines disease phenotype. J Immunol. 2007. 178:5578–5587.

19. Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008. 205:799–810.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download