Abstract
Background
EY-6 is one of the newly synthesized indoledione derivatives to induce tumor cell-specific cell death. In this study, we investigated the mechanism of immunological death induced by EY-6 at mouse colon cancer cell as well as at the normal immune cell represented by dendritic cell.
Methods
C57BL/6 mouse syngeneic colon cancer cell MC38 was treated with EY-6, and analyzed by MTT for viability test, flow cytometry for confirming surface expressing molecules and ELISA for detection of cytokine secretion. Normal myeloid-dendritic cell (DC) was ex vivo cultured from bone marrow hematopoietic stem cells of C57BL/6 mice with GM-CSF and IL-4 to analyze the DC uptake of dead tumor cells and to observe the effect of EY-6 on the normal DC.
Results
EY-6 killed the MC38 tumor cells in a dose dependent manner (25, 50 and 100 µM) with carleticulin induction. And EY-6 induced the secretion of IFN-γ but not of TNF-α from the MC38 tumor cells. EY-6 did not kill the ex-vivo cultured DCs at the dose killing tumor cells and did slightly but not significantly induced the DC maturation. The OVA-specific cross-presentation ability of DC was not induced by chemical treatment (both MHC II and MHC I-restricted antigen presentation).
The toxicity to the normal cell is the biggest limitation of anti-tumor therapy modules including chemotherapy and radiotherapy. Also as a systemic disease, tumor can't be treated or protected from metastasis by surgery which removes the local burden. To achieve the complete elimination of disease, one should consider the systemic minimal residual tumors (1). Thus, inducing specific immunity to remove the tumor is considered as the promising choice of therapy (2). Recent reports address the immunological aspects of certain chemotherapeutics (3-6). Generally chemotherapeutics kill the rapid proliferating cells including tumor cells as well as bone marrow stem cells which are the cause of immune-suppression in treated patients. High dose cyclophosphamide, a chemotherapeutics, inhibits T cell function and anthracyclines affect the macrophages (7-10). On the other hand, low dose cyclophosphamide induces the immunity. Unlike other anthracyclines, doxorubicin (10) did not inhibit but induce macrophage-related anti-tumor activity in vivo. Limited number of recent paper reports the immunological death of tumor cells killed by certain type of chemotherapeutics. Surface expression of carleticulin (CRT) or heat-shock proteins on the killed tumor cells leads to the induction of tumor-specific immune responses (1,11-13). Especially, translocation of cytosolic CRT onto the dead tumor cell surface makes the cell more attractive for uptake by antigen-presenting cell, DC. These findings allow us to make the hypothesis that a chemical inducing tumor cell specific and immunological killing may increases the tumor-specific immunity thus be a safe and effective anti-tumor agent.
The compound EY-6 is the newly synthesized indoledione derivatives with transposition of heterocylic ring (QIDs). Early study of related QIDs reveals that the compounds induce the tumor cell apoptosis by cell cycle control, angiogenesis control or topoisomerase II inhibition (14-17). In this study, the induction of immunological death of colon cancer cells by EY-6 is observed to learn the scientific basis to develop the candidate materials for efficacious and safe anti-cancer drug.
Specific pathogen-free female C57BL/6 mice (H2kb), 5~6 weeks old, were purchased from the Dae-Han BioLink (EumSung, Korea). The mice were provided with water and food, ad libitum and quarantined under 12 h light: 12 h dark photoperiod in the animal care facility of the Samsung Biomedical Research Institute, Samsung Medical Center, Seoul, Korea. Animal care was performed following the ILAR guideline. The mice were acclimated for at least one week before any experiments were conducted.
EY-6 was synthesized and supplied by Dr. Chung-Kyu Ryu (Ewha Women's University, Seoul, Korea). RPMI-1640 medium, fetal bovine serum and penicillin-streptomycin were obtained from GIBCO laboratories (Grand Island, NY, USA). Following antibodies for flow cytometric phenotyping were purchased from eBioscience (SanDiego, CA, USA); fluorescein isothiocyanate (FITC)-or phycoerythrin (PE)-labeled monoclonal Abs for FAS, HSP60, HSP90, HSP70, MHC class I (H2kb), CD8a, CD11c, CD80, CD11b (Mac1), and Gr-1. Antibody against CRT was obtained from ABCAM (Cambridge, UK). ELISA sets for cytokines including TNF-α and IFN-γ was purchased from eBioscience (SanDiego, CA, USA).
C57BL/6 syngeneic MC38, a colon carcinoma cell line was purchased from American type culture collection (ATCC) (Rockville, MD, USA). OVA-specific T cell hybridomas, B3Z86/90.14 (B3Z, MHC-I) and DOBW (MHC-II), were kindly provided by Dr. Kyungjae Kim (Sahm Yook University, Seoul, Korea). All the cell lines were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (complete medium) unless otherwise specified.
Mononuclear cells (MNCs) from bone marrow were obtained from the tibia and femur of cervical dislocated C57BL/6 mouse. Viability of red blood cell (RBC) free-MNCs was routinely over 90% by trypan blue exclusion. Plastic-adhered purified monocytes (1×106/ml) were incubated with GM-CSF and IL-4 (1×103 units/ml each) at 37℃ for 7 days in humidified CO2 incubator. Harvested DCs were used in following experiments: 1) EY-6 treated tumor cell uptake by DC, 2) analysis of the effects of EY-6 on cultured DC (cytotoxicity, phenotype, antigen-specific cross presentation).
The cells were cultured in the presence of EY-6 (25, 50 and 100 µM) for 24 h, 48 h, or 72 h in 96-well plates (1×104/well). After incubation at 37℃, modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT solution, 5 mg/ml, 20 µl) was added and incubated for 4 h at 37℃. At the end of the incubation, supernatant was removed and the color change induced by Dimethyl sulfoxide (DMSO) was determined at 540 nm with ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
EY-6 treated MC38 colon cancer cells stained with fluorescein isothiocyanate (FITC)-or phycoerythrin (PE)-labeled monoclonal Abs against FAS, HSP60, HSP90, HSP70, and CRT to analyse the immunogenicity induction. Cultured BM-DC characterization was performed after EY-6 treatment by staining with FITC or PE-conjugated mAb against CD11c, CD8a, H2b (MHC I), CD11b, Gr-1, CD80 (B7.1). Stained cells were analysed on the FACS Calibur (BD Biosciences, SanJose, CA, USA) within 3 hrs after the staining.
EY-6 treated tumor cell (MC38) was labeled with CRT-FITC then co-cultured with CD11c-PE labeled DC for 6 hr. Flow cytometric analysis was performed by FACS Calibur (BD Biosciences, SanJose, CA, USA). FITC and PE double positive cells were considered as DCs taken tumor cells.
Cultured BM-DCs were treated with different concentrations of EY-6 for overnight (1×105/well). DCs were added with OVA-peptide (257-264, SIINFEKL) (Peptron, Daejeon, Korea) for 2 h incubation at 37℃. After the washing twice with PBS, cells were fixed with 100 µl/well of ice-cold 1% paraformaldehyde for 5 min at room temperature. MHC I-restricted OVA-specific B3Z cells were (2×105/well) co-cultured with above DCs for 4 h at 37℃. After incubation, lacZ activity was measured by colorimetric analysis of freeze-thaw lysed cells with β-galactosidase substrate, cholorphenol red β-D-galactopyranoside (Calbiochem, Darmstadt, Germany) with ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
EY-6 treated and OVA peptide (323-339, ISQAVHAAHAEINEAGR) (Peptron, Daejeon, Korea)-introduced-DCs were co-cultured with MHC II-restricted DOBW cells (1×105/well). After 24 h incubation at 37℃, the plate was centrifuged at 1,800 rpm to collect the supernatant. OVA-specific secretion of IL-2 was measured in the supernatant by ELISA.
MTT assay was performed to observe the differential cytotoxic effect of EY-6 against the tumor cell and normal immune cells. MC38, a mouse colon cancer cell line was killed by EY-6 in dose-dependent manner (37.5% vs. 30.1% vs. 21.3% of non-treated control viability, for 48 hr exposure to 25, 50 and 100 µM EY-6, respectively) (Fig. 1A). Chemical could induce the MC38 apoptosis even with short time (18 hr) of lower dose (15 µM) exposure as determined by 26.3% annexin V+PI+ apoptotic MC38 cells. However the cultured normal BM-DC viability (136.6% vs. 127.0% vs. 157.6% of non-treated control viability, for 48 hr exposure to 25, 50 and 100 µM EY-6, respectively) was not affected or slightly proliferated by EY-6 (Fig. 1B). Following studies to define the EY-6 induced-immunological death were performed with 25 µM dose.
To observe the ability of EY-6 inducing immunological death of tumor cells, chemical treated tumor ce ll surface molecule expression was analyzed by flow cytometry. Death receptor Fas (CD95) expression was increased on the chemical-treated tumor cells (28.9% vs. 79.2% for the non-treated control vs. EY-6 treated MC38, respectively) (Fig. 2). Also the expression of natural adjuvant for immune response, heat shock proteins (HSPs), was induced on the tumor cell surface (7.5% vs. 80.6% for Hsp60; 2.5% vs. 28.4% for Hsp70; 16.1% vs. 40.6% for Hsp90 as control vs. EY-6 treated MC38, respectively) (Fig. 2). The most significant molecule speaks for the increased immunogenicity, CRT expression (20.7% vs. 50.4% for control vs. EY-6 treated MC38, respectively) was induced by EY-6 (Fig. 2). Thus chemical-treated tumor cells may be uptaken and presented to the immune system by DC, more easily than non-treated cells.
MC38 cell surface expression of Fas, Hsps, and CRT molecules, which are known to be responsible for the increased immunogenicity of dead cells, was significantly induced by EY-6 (Fig. 2). Then to observe the DC uptake of MC38 cells, it was observed that the CRT+CD11c+ double positive proportion in the co-culture of EY-6 treated MC38 cell and CD11c+ cultured-DC. EY-6 treatment significantly induced DC uptake of MC38 tumor cells (9.4% vs. 22.4% for non-treated control vs. ET-6 treated MC38, respectively) (Fig. 3). EY-6 induced CRT expression on the MC38 surface and DC uptake of these cells were proportionally co-related.
EY-6 treatment induced the secretion of IFN-γ from the MC38 cells (Fig. 4). Secretion of an inflammatory cytokine TNF-α was also increased but the absolute level was very low (78.3±7.2 vs.17.1±2.1 pg/ml at 6 h exposure to 25 µM EY-6 for IFN-γ vs. TNF-α) (Fig. 4). Data indicating that EY-6 treatment altered the tumor cell microenvironment favorable to anti-tumor immune responses.
Cultured-DC phenotype was observed by flow cytometry to see if EY-6 directly induced the cultured DC maturation. Unlike the killing effect on the MC38 tumor cells, cultured-DCs were not killed by EY-6 treatment rather, the cell proliferation induced slightly (Fig. 1B). Phenotype analysis of DC indicated that the Ey-6 did not induce DC maturation as was observed by decreased surface expression of CD80 (53.3% to 41.1% after EY-6 treatment), and CD11c (48.8% to 37.1% after EY-6 treatment) (Fig. 5). However, myeloid derived suppressor cell (MDSC) proportion was clearly reduced by EY-6 treatment (26.1% to 2.1% in EY-6 treated cells), suggesting the possible role of EY-6 on the myeloid cell differentiation (Fig. 5).
Cultured-DC presented OVA antigen to the OVA-specific T cell hybridomas either by MHC I-restricted (B3Z, CD8+ cell) and MHC II-restricted (DOBW, CD4+ cell) manner. MHC II-restricted OVA-specific IL-2 secretion from the DOBW cell was not significantly changed by the co-culture with EY-6 treated and OVA-introduced DC (270.4, 277.5±35.3, and 295.6±5.5 pg/ml with non-treated DC control, EY-6 25 µM and 50 µM treated-DC, respectively) (Fig. 6A). Also MHC I restricted OVA-specific B3Z cell response by EY-6 treated DC was not significant, neither (Fig. 6B). As whole, EY-6 treatment did not alter the cross-presentation ability of DCs for both MHC I and MHC II-restricted antigens.
EY-6 is newly synthesized indoledione derivatives with expectation of anti-fungal agent by an author (Dr. Chung-Kyu Ryu). Interestingly, in our screening test, tumor-specific killing by EY-6 without affecting normal immune cell viability was observed. Rather, the proliferation of normal splenocytes was increased by EY-6 in dose-dependent manner (data not shown). With an expectation for developing anti-tumor agent having immune-stimulatory effect, the influence of EY-6 on the MC38 mouse colon cancer cell line and normal mouse myeloid-DC was observed. MC38 colon cancer cell was killed by EY-6 in dose-dependent manner (Fig. 1A). It has been reported that some of the chemotherapeutics with certain doses induce the tumor cell apoptosis with increased surface expression of CRT. CRT is a cytosolic calcium-binding protein and expressed on the apoptotic tumor cell surface by certain type of chemotherapeutics such as anthracyclines. Surface expressed CRT work as an eat-me signal to DCs to induce tumor antigen-specific immune responses (11-13). Thus, this phenomenon called chemotherapeutics induced "immunological death" of tumor cell. In this study, tumor specific killing by chemical EY-6 was tested if it can induce the "immunogenic tumor cell death". EY-6 induced MC38 cell surface expression of not only CRT but also natural adjuvant Hsp 60s, 70s and 90s which are considered as immunogenicity-related molecules (Fig. 2). The CRT expression and the DC uptake of these dead cells were proportionally co-related (Fig. 3) meaning EY-6 can induce immunological death of MC38 tumor cells. Interestingly, EY-6 stimulated MC38 tumor cells to produce IFN-γ much more than TNF-α (Fig. 4). In general, IFN-γ is known to be produced by T cells or NK cells not by tumor cells. Immunotherapy with IFN-γ secreting tumor cells were proven to be effective cancer vaccine in animal model (18,19), suggesting that EY-6-manipulated IFN-γ secretion from the MC38 cells may be one of the important mechanism for inducing anti-tumor responses. Unlike the anti-tumor immune effect on the tumor cells, EY-6 did not affect normal antigen presenting cell, DC maturation or cross presentation function (Fig. 5 and 6). Together with the fact that EY-6 do not kill the normal DC, data confirm the tumor cell specific response of the chemicals. Data observed in this study suggest the possibility of developing EY-6 as a chemotherapeutics to kill the tumor cells specifically without toxicity to normal cells and manipulate the host immunity favorable to eliminate the tumor cells by inducing immunological death.
Figures and Tables
 | Figure 1EY-6 induced tumor-specific killing. MC38 cell (A) or cultured BM-DC (B) was treated with different doses of EY-6 (25, 50 and 100 µM) for 48 hr at 37℃. At the end of the incubation, supernatants were removed and MTT solution was added to analyze the cell death. Asterisks indicate the statistical significance at p<0.05. |
 | Figure 2Surface expression of immunogenicity-inducing molecules on the tumor cells killed by EY-6. MC38 cells were overnight incubated with or without EY-6 25 µM at 37℃. Then cells were stained with FITC or PE-tagged antibodies against surface molecules as was described in the "Materials and Methods" section. |
 | Figure 3Increased DC uptake of EY-6 treated MC38 cells. MC38 cells were overnight incubated with or without EY-6 25 µM at 37℃, then stained with CRT-FITC. Cultured-DC was stained with CD11c-PE. Two cells were co-incubated for 6 hr at 37℃ before observe the proportion of tumor cell uptake DCs by measuring CD11c+CRT+ double positive cells. |
 | Figure 4Cytokine secretion from the EY-6 treated MC 38 cells. MC38 cells were treated with EY-6 25 µM at 37℃. At several incubation time points, supernatants were obtained to measure the secreted cytokines (IFN-γ or TNF-α) by ELISA following manufacturer's protocol. Asterisks indicate the statistical significance at p<0.05. |
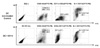 | Figure 5Effect of EY-6 on the DC maturation. Cultured BM-DC by the methods described in the "Materials and Methods" section, was treated with 25 µM EY-6 for 18~24 hr at 37℃. At the end of the incubation, harvested and stained the cells with FITC or PE-tagged antibodies for the surface molecules to identify DCs. |
 | Figure 6Effect of EY-6 on the DC for the OVA-specific cross presentation to CD4+(MHC II) and CD8+(MHC I) T cells. Cultured BM-DC was treated with EY-6 25 µM for overnight, then introduced with OVA before start the co-culture with MHC-restricted and OVA-specific T cell hybridomas. Details of methods are described in the "Materials and Methods" section. (A) IL-2 (pg/ml) secretion from the MHCII-restricted DOBW cells were measured by ELISA. (B) Color change induced by lacZ activity in the MHC I-restricted B3Z cells was measured. Numbers in Y-axes represent the absorption at OD580 nM. None of the responses were statistically significant. |
ACKNOWLEDGEMENTS
The authors sincerely thank to Dr. Kyung Jae Kim at the Sahm Yook University, Seoul, Korea for providing B3Z86/90.14 and DOBW T cell hybridomas. This study was supported by the grant from National Research Foundation of Korea (#2010-0004605).
References
1. Obeid M, Panaretakis T, Tesniere A, Joza N, Tufi R, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Leveraging the immune system during chemotherapy: moving calreticulin to the cell surface converts apoptotic death from "silent" to immunogenic. Cancer Res. 2007. 67:7941–7944.

2. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006. 313:1960–1964.

3. Apetoh L, Mignot G, Panaretakis T, Kroemer G, Zitvogel L. Immunogenicity of anthracyclines: moving towards more personalized medicine. Trends Mol Med. 2008. 14:141–151.

4. Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008. 8:59–73.

5. Chan OT, Yang LX. The immunological effects of taxanes. Cancer Immunol Immunother. 2000. 49:181–185.

6. Spreafico F, Vecchi A, Colotta F, Montovani A. Cancer chemotherapeutics as immunomodulators. Springer Semin Immunopathol. 1985. 8:361–374.

7. North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982. 155:1063–1074.

8. Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002. 62:2353–2358.
9. Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005. 11:6713–6721.

10. Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001. 61:3689–3697.
11. Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010. 16:3100–3104.

12. Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009. 28:578–590.

13. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Métivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007. 13:54–61.

14. Ko JH, Yeon SW, Ryu JS, Kim TY, Song EH, You HJ, Park RE, Ryu CK. Synthesis and biological evaluation of 5-arylamino-6-chloro-1H-indazole-4,7-diones as inhibitors of protein kinase B/Akt. Bioorg Med Chem Lett. 2006. 16:6001–6005.

15. Chung KH, Hong SY, You HJ, Park RE, Ryu CK. Synthesis and biological evaluation of 5-arylamino-1H-benzo[d]imidazole-4,7-diones as inhibitor of endothelial cell proliferation. Bioorg Med Chem. 2006. 14:5795–5801.

16. Kim JS, Rhee HK, Park HJ, Lee IK, Lee SK, Suh ME, Lee HJ, Ryu CK, Choo HY. Synthesis of 6-chloroisoquinoline-5,8-diones and pyrido[3,4-b]phenazine-5,12-diones and evaluation of their cytotoxicity and DNA topoisomerase II inhibitory activity. Bioorg Med Chem. 2007. 15:451–457.

17. Seo JM, Jin YR, Ryu CK, Kim TJ, Han XH, Hong JT, Yoo HS, Lee CK, Yun YP. JM91, a newly synthesized indoledione derivative, inhibits rat aortic vascular smooth muscle cells proliferation and cell cycle progression through inhibition of ERK1/2 and Akt activations. Biochem Pharmacol. 2008. 75:1331–1340.

18. Esumi N, Hunt B, Itaya T, Frost P. Reduced tumorigenicity of murine tumor cells secreting gamma-interferon is due to nonspecific host responses and is unrelated to class I major histocompatibility complex expression. Cancer Res. 1991. 51:1185–1189.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download