Abstract
Background
Traditionally, interferon-γ (IFN-γ) was regarded as a pro-inflammatory cytokine, however, recent reports suggested role of IFN-γ in immune tolerance. In our previous report, we could induce tolerance to male antigen (HY) just by male islet transplantation in wild type C57BL/6 mice without any immunological intervention. We tried to investigate the influence of IFN-γ deficiency on tolerance induction by male islet transplantation.
Methods
To examine the immunogenicity of male tissue in the absence of IFN-γ, we transplanted male IFN-γ knock-out (KO) skin to female IFN-γ KO mice. Next, we analyzed male IFN-γ KO islet to streptozotocin-induced diabetic female IFN-γ KO mice. And, we checked the functionality of grafted islet by graft removal and insulin staining.
Results
As our previous results in wild type C57BL/6 mice, female IFN-γ KO mice rejected male IFN-γ KO skin within 29 days, and did not reject male IFN-γ KO islet. The maintenance of normal blood glucose level was dependent on the presence of grafted male islet. And the male islet recipient did not reject 2nd challenge of male islet graft also.
It has been known that the skin graft across minor histocompatibility of male antigen (HY) mismatch is normally rejected (1,2). However, in our previous report, islet transplantation across HY barrier without any immunological manipulation induced HY antigen specific immunological tolerance which prevented the rejection of subsequent skin graft in C57BL/6 mouse model (3). In that tolerance, increased Foxp3+ regulatory T cells (Treg) mediated the tolerance induction. To explore the factor that made Treg increase and tolerance induction caused by islet transplantation, we started subtraction study using knock-out (KO) mouse. Our first choice was interferon-γ (IFN-γ) KO mouse.
IFN-γ was regarded traditionally as a pro-inflammatory cytokine. However its protective role in Th-1 cell-associated autoimmune diseases has recently been reported (4). For instance, ablation of IFN-γ resulted in aggravation of murine autoimmune disease, collagen-induced arthritis (CIA), indicating that endogenous IFN-γ inhibits important inflammatory pathway (5,6). The protective effects of endogenous IFN-γ also have been reported in other autoimmune models such as experimental autoimmune encephalomyelitis (EAE), experimental autoimmune uveitis (EAU), autoimmune nephritis, and myocarditis (7-13).
In Treg development, activation of signal transducer and activator of transcription-1 (STAT-1) seems to have a role (14). This suggests that cytokine, such as IFN-γ, that activate STAT-1 have a role in Treg development (4). Wang et al. (15) have reported that in vitro treatment of CD4+CD25- T cells with IFN-γ leads to their conversion to Foxp3+ Treg cells. And, copolymer-I induced in vitro Treg conversion was dependent on IFN-γ (16). In addition, Feng et al.(17) reported that ex vivo IFN-γ conditioning of CD4+ T cells with immature dendritic cells resulted in the Treg conversion, and these induced Treg cells could prevent allograft rejection. Another study in mice showed that IFN-γ expression in Treg cells is transiently up-regulated after alloantigen exposure in vivo and is important for their regulatory function (18). In another study, in vitro activated Treg cells have shown to produce IFN-γ and to also stimulate dendritic cells to produce indoleamine 2,3-dioxygenase (IDO) (19). This enzyme converts the essential amino acid tryptophan into N-formylkynurenine (20). By depleting tryptophan, IDO exerts the capacity to inhibit T-cell response. Recent evidence suggests an important role of IDO in the induction of tolerance (21).
Considering roles of IFN-γ in self-tolerance and Treg development, we speculated that deficiency of IFN-γ might result in the failure of tolerance induction in our male islet transplantation model. We examined immunogenicity of male skin in IFN-γ KO mice, and transplanted male islets to IFN-γ KO mice. By these experiments, we confirmed the role of IFN-γ in tolerance induction by male islet transplantation.
C57BL/6-Ifngtm1Ts(IFN-γ knock-out) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All IFN-γ KO mice used in this study were genotyped for IFN-γ. PCR was done with primers and cycling conditions recommended by The Jackson Laboratory. The normal endogenous gene was detected with forward primer IMR126 (5'-AGA AG T AAG TGG AAG GGC CCA GAA G-3') and reverse primer IMR127 (5'-AGG GAA ACT GGG AGA GGA GAA ATA T-3'). The mutant (knock-out) IFN-γ was detected with forward primer IMR128 (5'-TCA GCG CAG GGG CGC CCG GTT CTT T-3') and reverse primer IMR129 (5'-ATC GAC AAG ACC GGC TTC CAT CCG A-3'). Endogenous IFN-γ resulted in a 220-bp PCR product and mutant IFN-γ resulted in a 375-bp PCR product. Mice were bred and housed in a specific pathogen-free facility. Animal studies were conducted under protocols approved by Seoul National University Institutional Animal Care and Use Committee.
To induce diabetes mellitus, mice were injected intra-peritoneally with 125 mg/kg streptozotocin (Sigma, St. Louis, MO, USA) in two consecutive days. After the injections, non-fasting blood glucose levels were monitored using a glucometer, OneTouch Ultra (LifeScan Inc., Milpitas, CA, USA) from blood obtained by tail snipping. Mice with two consecutive non-fasting blood glucose levels higher than 250 mg/dl were considered as hyperglycemic mice. Hyperglycemic mice were selected for the islet transplantation.
Sacrificed donor mice's pancreases were injected with Hanks balanced salt solution containing 0.5 mg/ml of Collagenage P (Roche, Mannheim, Germany) via pancreatic duct. Inflated pancreases were excised and incubated in a 37℃ water bath for 20 minutes. Digested pancreases were filtered through a sieve and washed. Then, islets were purified from digests using Euro-Ficoll gradients. Obtained islets were re-suspended with RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 50µg/ml gentamycin, 100µM non-essential amino acids (all purchased from Invitrogen). After overnight culture, islets were used for the transplantation. Recipient mice were anesthetized with Isoflurane. Then, the left kidney was exposed and 250~300 islet equivalent number (IEQ) islets were delivered beneath the renal capsule.
Full-thickness tail skins obtained from donors were transplanted to the anesthetized recipient mice's graft beds on the left flank and covered with Vaseline gauze and Band-Aid (Johnson&Johnson, New Brunswick, NJ, USA). Bandages were removed after 7 days, and grafts were observed every 2 to 3 days for 3 week, and weekly thereafter. The graft was scored as rejected when less than 10% of viable tissue remained and visible inflammation ended.
Islet recipient's left kidney was removed under the anesthesia to confirm the graft function and to analyze graft histology. Removed kidney was frozen embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA). 5µm frozen section was done using Leica CM1850 cryocut microtome (Leica, Wetzlar, Germany). Acetone-fixated sections were stained with the hematoxylin and eosin. For insulin staining, sections were treated with guinea pig anti-insulin antibody (Dako Cytomation, Glostrup, Denmark) for 1 hr at room temperature. After wash with PBS, sections were treated with Alexa Fluor 555-conjuated anti-guinea pig IgG antibody (Invitrogen) for 1 hr at room temperature. Fluorescence microscopy was performed using Carl Zeiss Axio Imager A1 (Carl Zeiss, Jena, Germany). Images were analyzed using AxioVision software (Carl Zeiss).
In our previous study, wild type C57BL/6 female mice rejected male skin because of the minor antigen (HY) disparity (3). To test whether the absence of interferon-γ (IFN-γ) has an influence on the immunogenicity of male skin, we obtained skin from male IFN-γ knock-out (KO) mouse and transplanted it to female IFN-γ KO mice. Female recipients rejected male skin within 29 days (Fig. 1A). On the other hand, the recipients successfully accepted co-transplanted female skin. We could observe new hair growth of grafted female skin (Fig. 1B). Therefore, non-acceptance of male skin was not technical failure but rejection.
Wild type C57BL/6 female mice did not reject male islet and accepted subsequently grafted male skin (3). To test whether the absence of IFN-γ has an influence on the tolerance induction by male islet transplantation, we performed same male islet transplantation in the IFN-γ KO model. Islets were obtained from male IFN-γ KO mice, and transplanted to the left kidney subcapsular area of streptozotocin-induced diabetic female IFN-γ KO mice. Male islet transplantation normalized high blood glucose level of recipients, and the recipients did not reject male islet for >42 days (Fig. 2A).
To rule out the possibility of recipient's endogenous islet regeneration, we removed transplanted male islet in the left kidney by nephrectomy. Three days after the removal of transplanted islet, recipient returned to the hyperglycemic state (Fig. 2B). Therefore, the maintenance of normal blood glucose level was not because of the endogenous islet regeneration, but was dependent on the presence of transplanted male islet. To re-normalize the blood glucose level, and to check the recipient's response to 2nd antigen challenge, we again transplanted male IFN-γ KO islets to the recipient. 2nd islet transplantation promptly re-normalized the hyperglycemia, and the recipient maintained normal blood glucose level for >32 days (Fig. 2B). Because recipient did not reject 2nd male islet, we could conclude that non-rejection of 1st male islet was not just adaptation, and 1st male islet transplantation did not provoked immunological memory to male antigen.
To confirm whether the grafted islets were functioning, we analyzed the insulin secretion by grafted islet using the insulin staining of the graft site. Removed kidney containing grafted islet of recipient in Fig. 2B was frozen-sectioned and stained with hematoxylin & eosin to identify the position of graft site (Fig. 3A). Insulin staining was also conducted on the serial section. We could detect plenty of insulin-specific spots in islet region (Fig. 3B). Indicating that grafted islets were actively secreting insulin. As a control staining, only secondary Alexa Fluor 555-conjugated anti-guinea pig IgG antibody staining without primary anti-insulin antibody staining, did not produced any positive signal (Fig. 3C). Therefore, we could exclude the possibility of non-specific secondary antibody binding in Fig. 3B.
Role of IFN-γ in self tolerance was occasionally reported in autoimmune disease models. However its role in transplantation tolerance is not yet reported. As both the autoimmune disease studied and the graft rejection are mediated by Th1 dependent immune responses. We speculated that IFN-γ could have a role in transplantation tolerance, too. Our male islet transplantation model is unique in that transplantation of a graft without any immunological intervention induced antigen specific T cell tolerance. Therefore, we tried to check the role of IFN-γ in transplantation tolerance using our male islet transplantation model.
IFN-γ has been known to play a very important role in graft rejection (22), we first tested whether the absence of IFN-γ in the IFN-γ KO mouse could affect the consequences of skin and islet transplantation across HY barrier. Despite the absence of IFN-γ, female IFN-γ KO mice rejected male IFN-γ KO skin and accepted male IFN-γ KO islet as was observed in the previous results in wild type C57BL/6 model.
Although results of male skin and islet transplantation were the same as those in WT mice, the fate of male skin graft in the recipient harboring prior male islet graft should be checked to confirm the induction of tolerance. However, 2nd islet transplantation result indirectly suggests the possibility of tolerance induction. If the 1st islet transplantation provoked normal immune response to male antigen, there should be accelerated immune response by the induction of immunological memory. However, the recipient accepted 2nd challenge of male islet graft indicating the recipient got tolerized to male antigen of HY.
Our investigation on the role of IFN-γ in this study could not be generalized to transplantation tolerance. To induce transplantation tolerance, many investigators used co-stimulatory blockade or co-receptor blockade regimens (23-27). However our male islet transplantation induced tolerance without any immunological manipulation. Although our method also induced transplantation tolerance, this unique trait distinguished our method from other conventional methods. So, it is hard to say that our model is a representative of general tolerance induction model. Therefore, further investigations are required to confirm the role of IFN-γ in transplantation tolerance.
As an effort to delineate the underlying mechanisms of immune tolerance induced by the male islet transplantation, we checked the possible involvement of IFN-γ. The achievement of immunological tolerance across HY barrier in IFN-γ KO mouse suggests that IFN-γ would not be the key factor for the islet transplantation mediated tolerance induction. Further study should be needed to see if other factors known to be required for tolerance induction, such as TGF-β (28), PD-L1 (29) would be involved in the process of tolerance induction.
Figures and Tables
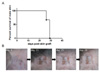 | Figure 1Male skin rejection in interferon-γ knock-out mice. Full-thickness tail skins obtained from male interferon-γ knock-out (IFN-γ KO) mice were transplanted to female IFN-γ KO mice (n=3). (A) Percent survival of the transplanted male skins is depicted. All recipients rejected male skin within 29 days. Mean survival time was 28 days. (B) One representative recipient's photos of grafted skins are arrayed in serial order. Days after skin graft (8, 21, 25, 28) are marked on the upper-left corners of each photos. The left grafted skin on the photo is female skin and the right one is male skin. New black hair growth on upper part of female skin can be seen. On the other hand, male skin had inflammation and disappeared and black hairs on lower part also disappeared. |
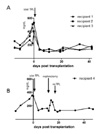 | Figure 2Male islet transplantation in IFN-γ knock-out mice. Female IFN-γ KO mice were rendered diabetic by 2 times of 125 mg/kg streptozotocin intraperitoneal injection. Non-fasting blood glucose levels were monitored. Male IFN-γ KO islet transplantations were operated to the diabetic recipients. Blood glucose levels (mg/dl) during the transplantation period are depicted. Each line and points indicates individual recipients. (A) Male islet transplantation normalized hyperglycemia of recipients. Recipients maintained normal blood glucose levels for >42 days (n=3). Arrow indicates the time point of male islet transplantation. (B) After the male islet transplantation to recipient's left kidney, grafted islet was removed by nephrectomy. After the re-surge of blood glucose level, 2nd male islet transplantation was done and normalized the hyperglycemia again. Arrows indicates the time points of 1st male islet transplantation, nephrectomy and re-transplantation, respectively. |
 | Figure 3Histological analysis of islet graft. Removed kidney containing grafted islet of recipient in Fig. 3B was frozen-sectioned as 5µm thickness and acetone-fixated. (A) H&E staining was done to the section. Rectangular area indicates islet region. (B) Insulin staining was done with primary guinea pig anti-insulin antibody and secondary Alexa Fluor 555-conjugated anti-guinea pig IgG antibody, subsequently. Red spots indicate plenty of stained insulin in islet region. Blue spots are nuclei of cells resulted by DAPI staining. (C) As a control of '(B)', secondary antibody staining without primary antibody did not result in non-specific red spots. Magnifications are ×100. |
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Healthcare technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Project No. A040004).
References
1. Millrain M, Chandler P, Dazzi F, Scott D, Simpson E, Dyson PJ. Examination of HY response: T cell expansion, immunodominance, and cross-priming revealed by HY tetramer analysis. J Immunol. 2001. 167:3756–3764.

2. Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen, H-Y: a history of transplantation, immune response genes, sex determination and expression cloning. Annu Rev Immunol. 1997. 15:39–61.

3. Yoon IH, Choi SE, Kim YH, Yang SH, Park JH, Park CS, Kim Y, Kim JS, Kim SJ, Simpson E, Park CG. Pancreatic islets induce CD4(+) [corrected] CD25(-)Foxp3(+) [corrected] T-cell regulated tolerance to HY-mismatched skin grafts. Transplantation. 2008. 86:1352–1360.

4. Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check. Trends Immunol. 2008. 29:479–486.
5. Manoury-Schwartz B, Chiocchia G, Bessis N, Abehsira-Amar O, Batteux F, Muller S, Huang S, Boissier MC, Fournier C. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J Immunol. 1997. 158:5501–5506.
6. Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J Immunol. 1997. 158:5507–5513.
7. Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988. 140:1506–1510.
8. Caspi RR, Chan CC, Grubbs BG, Silver PB, Wiggert B, Parsa CF, Bahmanyar S, Billiau A, Heremans H. Endogenous systemic IFN-gamma has a protective role against ocular auto-immunity in mice. J Immunol. 1994. 152:890–899.
9. Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M. Dual role of the IL-12/IFN-gamma axis in the development of autoimmune myocarditis: induction by IL-12 and protection by IFN-gamma. J Immunol. 2001. 167:5464–5469.

10. Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996. 156:5–7.
11. Jones LS, Rizzo LV, Agarwal RK, Tarrant TK, Chan CC, Wiggert B, Caspi RR. IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J Immunol. 1997. 158:5997–6005.
12. Ring GH, Dai Z, Saleem S, Baddoura FK, Lakkis FG. Increased susceptibility to immunologically mediated glomerulonephritis in IFN-gamma-deficient mice. J Immunol. 1999. 163:2243–2248.
13. Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996. 157:3223–3227.
14. Nishibori T, Tanabe Y, Su L, David M. Impaired development of CD4+ CD25+ regulatory T cells in the absence of STAT1: increased susceptibility to autoimmune disease. J Exp Med. 2004. 199:25–34.

15. Wang Z, Hong J, Sun W, Xu G, Li N, Chen X, Liu A, Xu L, Sun B, Zhang JZ. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25-T cells to CD4+ Tregs. J Clin Invest. 2006. 116:2434–2441.
16. Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci U S A. 2005. 102:6449–6454.

17. Feng G, Gao W, Strom TB, Oukka M, Francis RS, Wood KJ, Bushell A. Exogenous IFN-gamma ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur J Immunol. 2008. 38:2512–2527.

18. Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005. 201:1925–1935.

19. Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003. 4:1206–1212.

20. Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003. 81:247–265.

21. Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004. 4:762–774.

22. Nast CC, Zuo XJ, Prehn J, Danovitch GM, Wilkinson A, Jordan SC. Gamma-interferon gene expression in human renal allograft fine-needle aspirates. Transplantation. 1994. 57:498–502.

23. Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004. 172:6003–6010.

24. Lechler RI, Garden OA, Turka LA. The complementary roles of deletion and regulation in transplantation tolerance. Nat Rev Immunol. 2003. 3:147–158.

25. Li XC, Strom TB, Turka LA, Wells AD. T cell death and transplantation tolerance. Immunity. 2001. 14:407–416.

26. Scully R, Qin S, Cobbold S, Waldmann H. Mechanisms in CD4 antibody-mediated transplantation tolerance: kinetics of induction, antigen dependency and role of regulatory T cells. Eur J Immunol. 1994. 24:2383–2392.

27. Waldmann H, Cobbold S. Regulating the immune response to transplants. A role for CD4+ regulatory cells? Immunity. 2001. 14:399–406.
28. Zhang L, Yi H, Xia XP, Zhao Y. Transforming growth factor-beta: an important role in CD4+CD25+ regulatory T cells and immune tolerance. Autoimmunity. 2006. 39:269–276.

29. Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, Grimm M, Waaga AM, Ueno T, Padera RF, Yagita H, Azuma M, Shin T, Blazar BR, Rothstein DM, Sayegh MH, Najafian N. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007. 179:5204–5210.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download