This article has been corrected. See "Erratum to “Stimulating Effect of a Novel Synthesized Sulfonamido-Based Gallate ZXHA-TC on Primary Osteoblasts” by Jin P, et al. (Yonsei Med J 2015;56(3):760–771.)" in Volume 60 on page 704.
Abstract
Purpose
This study is intended to investigate the effects of plants or plant-derived antioxidants on prevention of osteoporosis through the maintenance of reactive oxygen species (ROS) at a favorable level.
Materials and Methods
In this study, a novel antioxidant, namely 3,4,5-Trihydroxy-N-[4-(5-hydroxy-6-methoxy-pyrimidin-4-ylsulfamoyl)-phenyl]-benzamide (ZXHA-TC) was synthesized from gallic acid and sulfadimoxine. Its effect on osteoblast metabolism was investigated via the detection of cell proliferation, cell viability, production of ROS, and expression of osteogenic-specific genes including runt-related transcription factor 2 (RUNX2), bone sialoprotein (BSP), osteocalcin (OCN), alpha-1 type I collagen (COL1A1), and osteogenic-related proteins after treatment for 2, 4, and 6 days respectively.
Results
The results showed that ZXHA-TC has a stimulating effect on the proliferation and osteogenic differentiation of primary osteoblasts by promoting cell proliferation, cell viability, and the expression of genes BSP and OCN. Productions of bone matrix and mineralization were also increased by ZXHA-TC treatment as a result of up-regulation of COL1A1 and alkaline phosphatase (ALP) at the early stage and down-regulation of both genes subsequently. A range of 6.25×10-3 µg/mL to 6.25×10-1 µg/mL is the recommended dose for ZXHA-TC, within which 6.25×10-2 µg/mL showed the best performance.
Osteoporosis, a public health concern, is a systemic skeletal disease characterized by micro-architectural deterioration of bone with resultant low bone mass, bone fragility, and increased fracture risk.1,2,3,4 As reported by the National Osteoporosis Foundation, osteoporosis affects approximately 10 million individuals5 and over 2 million fractures per year6 in the USA. It is estimated that up to 50% of women and 25% of men over the age of 50 years will experience an osteoporotic fracture in their remaining lifetime.5 Osteoporotic fractures can cause considerable pain, disability, loss of independence, depression, deterioration in quality of life, and even death.4,7,8 These negative effects not only impose a great economic burden on family and country but also cause great anxiety for the patients.
There is an increasing awareness that plants and plant-derived compounds may play an important role in the prevention of osteoporosis.9 Many bioactive compounds from plants, such as daidzein and its derivative,10,11,12,13,14 (-)-Epigallocatechin-3-gallate (EGCG),15 were found to act as estrogen receptor agonists with beneficial outcomes in osteoporosis. Their prevention of osteoporosis may be attributed partly to their antioxidant properties. In the process of osteoporosis, excessive reactive oxygen species (ROS) and inflammatory responses stimulate differentiation and function of osteoclasts and simultaneously suppress osteoblastic proliferation and differentiation.16 Thus, antioxidants that can maintain ROS at a favorable level by reducing excessive ROS may hold promise in the prevention of osteoporosis.
Gallic acid (GA) and its derivatives comprise a group of polyphenol compounds that have been known to affect several pharmacological and biochemical pathways and have strong anti-oxidative17 and anti-inflammatory18 effects. Additionally, GA has been reported to have an anti-osteoporosis effect.19 However, GA was reported to suppress cell proliferation20,21,22 and to be more hydrophilic than its esters,17 which may influence its anti-osteoporosis effect. Therefore, the introduction of certain lipophilic compounds may improve the bioactivity of GA and broaden its application. As recommended by Nuti, et al.,23 modification by sulfonamide groups may enhance the hydrophobicity and bioactivity of GA and therefore support the growth of cells. It is the character of sulfonamide to modify antibiotic ability and hydrophobicity by replacing hydrogen atoms on the para-position of the amino with a different heterocyclic structure.
In this study, we synthesized sulfonamido-based gallate ZXHA-TC and investigated its effect on the osteoblasts isolated from rat calvaria by detecting cell proliferation, the expression of osteogenic specific genes and proteins.
ZXHA-TC was prepared from GA and sulfadimoxine. The synthetic route is presented in Fig. 1 in detail. After the reactions, an appropriate amount of distilled water was added to the mixture, and the precipitated raw product was separated by vacuum filtration. The raw product was recrystallized in a tetrahydrofuran-methanol solvent system.
Primary osteoblasts were harvested from the bilateral parietal bone of 3 to 7 days' newborn Sprague Dawley rats by enzymatic digestion. After rats were put to death by cervical dislocation, the bilateral parietal bones were stripped clearly with sterile gauze in a sterile environment. After the connective tissue around the parietal bone was removed by 0.25% trypsin-ethylene diamine tetraacetic acid (EDTA) (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China), the bone was cut into pieces about 1×1 mm in a sterile vial and then digested with 1 mg/mL collagenase type I (Gibco-BRL, Carlsbad, CA, USA) in serum-free alpha-modified Eagle's medium (α-MEM, Gibco-BRL) for 3 h. After centrifugation at 1000 rpm for 5 min, isolated osteoblasts were suspended in α-MEM containing 10% (v/v) fetal bovine serum (FBS, Gibco-BRL) and 1% (v/v) antibiotics (penicillin 100 U/mL, streptomycin 100 U/mL). Cultures were maintained in a 5%-CO2 incubator (Thermo Scientific™ Forma Series II Water-Jacketed, Santa Ana, CA, USA) at 37℃ with the culture medium changed every other day. At 80-90% confluence after about 7 days of culture, primary cells were used for subsequent experiments.
ZXHA-TC was dissolved in sodium hydroxide solution (NaOH, Sigma, St. Louis, MO, USA), its PH value was adjusted to 7.0 as stock solution, and it was then stored at 4℃. The stock solution of ZXHA-TC was then added to the cell cultures in various concentrations. Culture medium containing these various concentrations of ZXHA-TC was replaced every other day.
To detect the effect of ZXHA-TC on primary osteoblasts, concentration screening was assessed by the 3-(4,5)-dimethy-lthiahiazo(-z-y1)-3,5-di-phenytetrazolium-romide (MTT; Sigma) method. Briefly, cells were detached by 0.25% trypsin-EDTA, suspended in α-MEM containing 10% (v/v) FBS and 1% (v/v) antibiotics (penicillin 100 U/mL, streptomycin 100 U/mL) and seeded into a 96-well plate at a density of 1×103 cells/well. Additionally, the outside circle wells were sealed with phosphate buffered saline (PBS). After 24 hours of culture, the medium was replaced with 200 µL culture medium containing ZXHA-TC ranging from 0 µg/mL to 2.56×105 µg/mL. The solution of MTT in PBS was added to each well with a final concentration of 5 mg/mL after 3 days of interference. After 4 hours of incubation, the supernatant was discarded, and 200 µL Dimethyl sulfoxide (DMSO, Sigma) was added for formazan-crystal solubilization. After continuous gentle shaking for 10 minutes in a dark environment to dissolve formazan-crystal thoroughly and evenly, the absorbance value was measured at 570 nm with a microplate reader (Thermo Scientific Multiskan GO Microplate Spectrophotometer, Helsinki, Finland). All samples were done in triplicate, and results were shown as absorbance values. The detection of cytotoxicity was started by seeding 5×103 cells/well in 24-well plates and then treating with the concentrations of ZXHA-TC that had been selected by the previous concentration screening assay. After 2, 4, and 6 days, a solution of MTT in PBS was added to each well, with a final concentration of 5 mg/mL. After 4 hours of incubation, the supernatant was discarded and 1 mL DMSO was added for formazan-crystal solubilization. After continuous gentle shaking for 10 minutes in the dark to dissolve the DMSO thoroughly and evenly, samples at 200 µL were randomly extracted from each of three parallel wells at the same concentration three times and transferred to 96-well plates. The absorbance value was measured at 570 nm with the microplate reader, and the results were shown as optical-density absorbance values.
Cell viability was determined by fluorescein diacetate [FDA; Life Technologies (AB & Invitrogen), Carlsbad, CA, USA]-propidium iodide [PI; Life Technologies (AB & Invitrogen)] staining at 2, 4, and 6 days. Briefly, FDA and PI stock solutions were added to the cells at final concentrations of 5 µg/mL and 20 µg/mL, respectively, and incubated in a dark environment for 5 min at 37℃. Images were captured and statistically analyzed via laser scanning confocal microscope (Nikon A1, Tokyo, Japan).
Cell proliferation was detected with a 5-bromo-2-deoxyuridine (BrdU) cell proliferation detection kit (Sigma), following the manufacturer's instructions. Briefly, primary osteoblasts were seeded in a 10-cm diameter culture dish. After 24 hours, cells were treated with ZXHA-TC at different concentrations for 4 days. Afterwards, cells were detached, suspended at a concentration of 1×106 cells in 500 µL per tube, fixed, rinsed, denatured, labeled with BrdU, and detected by flow cytometry (BD Biosciences, Newark, NJ, USA), with excitation and emission settings at 488 nm and 520 nm, respectively.
After being cultured for 2, 4, and 6 days, cells were washed with PBS two times and fixed with 4% paraformaldehyde (Cytoskeleton, Denver, CO, USA) for 10 minutes. Subsequently, the cells were treated with 0.5% Triton-X (Gibco-BRL) for 5 min and then incubated in a dark environment with rhodamine phalloidin (Sigma) for 30 min. Eventually, after rinsing with PBS, the cells were incubated away from light with Hoechst 33258 (Sigma) for 5 min. Images were captured and statistically analyzed by a laser scanning confocal microscope.
A real-time polymerase chain reaction (RT-PCR) assay was performed to detect the expression of runt-related transcription factor 2 (RUNX2), bone sialoprotein (BSP), osteocalcin (OCN) and alpha-1 type I collagen (COL1A1). At 2, 4, and 6 days, total RNA was extracted with an RNA extraction kit (Beijing ComWin Biotech Co., Ltd., Beijing, China) according to the manufacturer's instructions. An equal amount of RNA (300 ng) was used as a template and was reverse transcribed into cDNA using a reverse transcription kit (Fermentas company, Pittsburgh, PA, USA), which was then amplified by using an SYBR-Green mix kit (Roche company, Berlin, Germany) on a real-time fluorescence quantitative instrument (realplex 4, Eppendorf Corporation, Hamburg, Germany). The primers used for PCR were designed as shown in Table 1. The dissociation curve of each primer pair was analyzed to confirm the primer specificity. Marker gene expressions were analyzed by the 2-ΔΔCT method using β-actin. Each sample was repeated three times for each gene.
Alkaline phosphatase (ALP) activity assay and ALP staining were carried out using ALP detection reagent kit and ALP staining kit (both purchased from Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China) respectively following the manufacturer's instructions. After 2, 4, and 6 days of culturing primary osteoblasts, culture medium was collected and slices were fixed for staining. After being centrifuged at 2500 rpm for 10 min, the supernatant of the medium was harvested for subsequent assay. Briefly, after adding buffer solution, matrix solution, water bath, and coloring, the absorbance value was measured at 520 nm with the microplate reader. Subsequently, the activity value was calculated with a computational formula. All samples were done in triplicate. Slices were washed with PBS, and staining was processed following the manufacturer's instructions. The staining and subsequent related staining were all observed and photographed by an inverted phase contrast microscope (Olympus, Tokyo, Japan) and its related image acquisition system (Nikon, Tokyo, Japan).
Production of intracellular ROS was assayed with a ROS detection reagent kit (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China) according to the manufacturer's instructions. Briefly, primary osteoblasts were seeded in concentrations of 2×104 cells/well in 6-well plates and cultured with different concentrations of ZXHA-TC after 24 hours. All samples were done in triplicate. After 2, 4, and 6 days of treatment, culture medium was replaced with 10 µM 2,7-dichlorofuorescin diacetate and incubated for 30 min at 37℃ in the dark environment. After being washed with PBS three times, cells were detached using 0.25% trypsin-EDTA and suspended in a culture medium at a density of 1×105 cells/mL. Samples at 100 µL were randomly extracted from each of three parallel wells at the same drug concentration three times and transferred to 96-well plates. Oxidative burst was detected with a microplate fluorescence reader (FLx800, Biotek, Montpelier, VT, USA) with excitation and emission setting at 485 nm and 525 nm respectively.
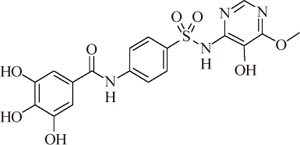
3,4,5-Trihydroxy-N-[4-(5-hydroxy-6-methoxy-pyrimidin-4-ylsulfamoyl)-phenyl]-benzamide, white crystals, mp: >270℃, ESI-MS: 447.1[M-H]-, 1H-NMR (400 MHz, DMSO-d6) δ 10.58 (s, 1H, -SO2-NH), 10.25 (s, 1H, -CO-NH), 7.93-7.86 (dd, J=9.0 Hz, 4H, 4×Ar-H), 7.78 (s, 1H, Py-H), 6.94 (s, 2H, 2×Ar-H), 3.67 (s, 3H, -OCH3); 13C-NMR (125 MHz, DMSO) δ 166.05, 157.98, 145.58, 144.00, 143.54, 137.31, 134.92, 129.85, 128.28, 124.40, 119.34, 107.44, 59.02.
As shown in Fig. 2A, ZXHA-TC indicated low cytotoxicity at the concentration ranging from 6.25×10-5 to 2.5×102 µg/mL and presented no significant differences with the control group from 5×102 to 2×103 µg/mL. Within this range, ZXHA-TC ranged from 6.25×10-3 to 6.25×10-1 µg/mL significantly promoted cell growth (p<0.05). In contrast, ZXHA-TC ranged from 4×103 to 2.56×105 µg/mL displayed an inhibitive effect on primary osteoblasts. Therefore, ZXHA-TC concentrations of 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL were chosen for further investigation.
As shown in Fig. 2B, cytotoxicity was treated with ZXHA-TC at different concentrations (0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL), and primary osteoblasts treated with ZXHA-TC grew significantly faster than that of the control in the same culture period. Among the three concentrations, 6.25×10-2 µg/mL exhibited the strongest promoting effect on cell growth.
Cell viability was determined by FDA-PI staining (Fig. 3), in which viable cells were stained green and dead cells were stained red. The results demonstrated that ZXHA-TC exerted a potent effect on primary osteoblast survival. The FDA-PI staining images indicated that there were more live cells in ZXHA-TC groups than in the control, which was consistent with the results of cell proliferation. The results implied that ZXHA-TC had a positive effect on cell growth. Among the experimental groups, a concentration of 6.25×10-2 µg/mL was superior to others.
To measure cell proliferation, cells were stained with anti-BrdU FITC and analyzed using flow cytometry. As shown in Fig. 4, compared with the control, ZXHA-TC has a positive effect on cell proliferation, especially at the concentration of 6.25×10-2 µg/mL.
The attachment and spreading of osteoblasts was assessed by detecting the actin filaments with rhodamine phalloidin and Hoechst 33258 staining. As shown in Fig. 5, the cells in ZXHA-TC-treated groups grew in clumps with densely-distributed extracellular matrix, which indicates that cells in these groups communicated more closely than those in the control. Particularly, a ZXHA-TC concentration of 6.25×10-2 µg/mL exhibited more distributed actin filaments than others.
The effect of ZXHA-TC on osteoblasts was further investigated through gene expression of RUNX2, BSP, OCN, and COL1A1 after culturing cells for 2, 4, and 6 days. As shown in Fig. 6, BSP and OCN were all notably promoted by ZXHA-TC at the three observation points. The results indicated that ZXHA-TC could up-regulate the expressions of genes BSP and OCN continuously. However, the expressions of genes RUNX2 and COL1A1 were up-regulated remarkably from 2 to 4 days and then down-regulated gradually thereafter. Among all groups, the concentration of 6.25×10-2 µg/mL performed better than others, as demonstrated by the highest expressions of BSP and OCN, both of which are osteogenic-specific marker genes.
As shown in Fig. 7A, despite no significant differences found between the ZXHA-TC-treated groups and the control at 2 days, primary osteoblasts treated with three concentrations all exhibited higher ALP activity than those in the control at 4 and 6 days. Overall, ALP activity tended to increase from 2 to 4 days and decrease slightly thereafter. This trend was further confirmed by ALP staining, shown in Fig. 7B-M. The quantitative and qualitative assays both demonstrated that ZXHA-TC was beneficial to osteogenic differentiation, and those at the concentration of 6.25×10-2 µg/mL performed prominently.
To detect the level of intracellular ROS, the microplate fluorescence reader was adopted to assess the intensity of fluorescence indirectly. As shown in Fig. 8, although no significant differences were found between the ZXHA-TC-treated groups and the control at 2 days, the level of intracellular ROS was significantly reduced compared with the control, and the concentration of 6.25×10-2 µg/mL performed outstandingly.
GA has been reported to have a strong antioxidant effect and is known to impact several pharmacological and biochemical pathways.17 However, its inferior bioactivity has limited its clinical usage.17,20 Moreover, the hydrophilicity of GA has hindered its application as a potent antioxidant, as appropriate hydrophobicity may be essential for promoting antioxidant abilities.17 GA derivatives were reported to have no toxicity to human erythrocytes even at higher concentrations.24 To improve the pharmacological effect and biological properties of GA,25,26,27,28 modification is of significance. In this study, ZXHA-TC was synthesized by coupling sulfonamide groups with GA, and the effects of ZXHA-TC on osteoblast growth and metabolism were investigated.
As evidenced by concentration screening and cytotoxicity assay, ZXHA-TC concentrations ranging from 6.25×10-5 to 2.5×102 µg/mL were found to show no cytotoxicity, with a promoting effect at the range of 6.25×10-3 to 6.25×10-1 µg/mL (Fig. 2). Further exploration of cell viability, cell proliferation, and PCR analysis revealed that a ZXHA-TC concentration of 6.25×10-2 facilitated cell growth the most in all groups (Figs. 3, 4, 5, 6).
Osteogenic specific genes RUNX2, BSP, OCN, and COL1A1 were evaluated to determine the effect of the compound on osteoblasts. Expressions of RUNX2 and COL1A1 were up-regulated remarkably from 2 to 4 days and down-regulated gradually from 4 to 6 days (Fig. 6). As the master regulator essential for osteoblast development and maturation, RUNX2 plays a crucial role in the early stage of bone calcification, favoring bone formation and calcification.29 However, over-expression of RUNX2 in the late stage inhibits osteoblast maturation.30,31 Thus, ZXHA-TC-oriented early up-regulation and subsequent down-regulation of RUNX2 may be beneficial to the overall process of osteogenic differentiation. COL1A1, known as a major specific marker of bone matrix, is highly expressed during bone formation32 though down-regulated in the process of osteoblast differentiation.33 The expression of COL1A1 increased in the early period (Fig. 6D) after treatment with ZXHA-TC, indicating the positive role of ZXHA-TC on the formation of bone matrix. As a result, BSP and OCN, which are specific markers in mineralized tissues,34,35,36,37 were up-regulated by ZXHA-TC continuously during the culture period (Fig. 6B and C), indicating that ZXHA-TC is beneficial to osteogenic differentiation and mineralization.
ALP produced by osteoblasts is found to be involved in the degradation of inorganic pyrophosphate, which provides sufficient local phosphate or inorganic pyrophosphate for mineralization to occur.32 Commonly used as a marker of osteogenesis, ALP activity increases during the differentiation stage and declines when mineralization occurs.38,39 In this study, ALP activity in the ZXHA-TC-treated groups increased early yet decreased later (Fig. 7A), contrary to the control, in which a continuous increase was observed during the whole period. This was further verified by ALP staining (Fig. 7B-M).
ROS have been appreciated as regulators of many cellular processes. However, over-expression of intracellular ROS may not only induce chromosomal damage40 but also suppress osteoblastic proliferation and differentiation.16 Thus, it is necessary to maintain ROS at a moderate level in order to benefit cellular processes. The maintenance of the ROS level may be essential in the prevention of osteoporosis. ZXHA-TC was found to have a prominent effect on reducing the level of intracellular ROS, especially at the concentration of 6.25×10-2 µg/mL (Fig. 8). All these results demonstrate that ZXHA-TC is beneficial to the overall process of osteogenic differentiation.
In summary, the effect of ZXHA-TC on primary osteoblasts was well-documented as verified by analysis of cell proliferation, cell viability, and RT-PCR results. The recommended dose of ZXHA-TC ranges from 6.25×10-3 to 6.25×10-1 µg/mL, particularly 6.25×10-2 µg/mL. The evidences found in this study indicate that ZXHA-TC may have a positive effect on osteogenic differentiation in vitro, implying it as a potential agent for the treatment of osteoporosis in a clinical application.
Figures and Tables
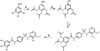 | Fig. 1The synthetic route of ZXHA-TC was presented. Reagents and conditions. (A) Acetyl oxide, oil bath, 120℃. (B) SOCl2, oil bath, 80℃. (C) Sulfadimoxine, THF, pyridine, ice bath. (D) HCl, THF, 60℃. |
 | Fig. 2MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was used to analyze the cytotoxicity of primary osteoblasts treated with ZXHA-TC at different concentrations. (A) A range of 0-2.56×105 µg/mL of ZXHA-TC was chosen. The result shows that ZXHA-TC at the concentrations ranging from 6.25×10-5 to 2.5×102 µg/mL showed low cytotoxicity, among which 6.25×10-3 µg/mL to 6.25×10-1 µg/mL have a significantly positive effect on cell viability (p<0.05). (B) The effect of ZXHA-TC at the concentrations of 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL on primary osteoblasts was detected (n=9). Different letters indicate that the two groups are significantly different from each other (p<0.05), and similar letters indicate no significant difference. Treatment of ZXHA-TC promoted cell viability in a dose-dependent manner; particularly at the concentration of 6.25×10-2 µg/mL, ZXHA-TC enhanced cell viability the most. *Significant difference (p<0.05, n=3). |
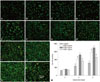 | Fig. 3Cell viability was determined by fluorescein diacetate-propidium iodide staining, in which viable cells were stained green and dead cells were stained red. (A-D) Staining of primary osteoblasts treated with ZXHA-TC at concentrations of 0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL at 2 days. (E-H) Staining of primary osteoblasts treated with ZXHA-TC at concentrations of 0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL at 4 days. (I-L) Staining of primary osteoblasts treated with ZXHA-TC at concentrations of 0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL at 6 days. (M) Statistical analysis of the data from the staining pictures from A-L (n=3). As time elapsed, more and more dead cells were found in each group. Comparatively, more viable cells were found in the ZXHA-TC-treated groups, which indicated the positive effect on the primary osteoblasts. Scale bar=200 µm. |
 | Fig. 4Cell proliferation was detected with a BrdU cell proliferation detection kit by flow cytometry. (A-D) Represent the results of primary osteoblasts treated with ZXHA-TC at concentrations of 0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL, respectively, at 4 days. The results showed that ZXHA-TC promoted cell growth markedly, especially at the concentration of 6.25×10-2 µg/mL. |
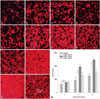 | Fig. 5Actin filament was detected with rhodamine phalloidin-Hoechst 33258 staining, in which the actin filament and nuclei were stained red and blue respectively. (A-D) Staining of primary osteoblasts treated with ZXHA-TC at concentrations of 0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL at 2 days. (E-H) Staining of primary osteoblasts treated with ZXHA-TC at concentrations of 0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL at 4 days. (I-L) Staining of primary osteoblasts treated with ZXHA-TC at concentrations of 0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL at 6 days. (M) Statistical analysis of the data from the staining pictures from A-L (n=3). Cells in ZXHA-TC-treated groups grew in clumps, with the density of the groups at the concentration of 6.25×10-2 µg/mL being most salient, which indicates that cells in these groups communicate more closely than those in the control. Scale bar=100 µm. |
 | Fig. 6A quantitative real-time polymerase chain reaction was used to analyze the progression of the expression of osteogenic genes RUNX2 (A), BSP (B), OCN (C), and COL1A1 (D) in primary newborn osteoblasts cultured in different groups for 2, 4, and 6 days. Compared with the control, expressions of the four genes were all up-regulated. However, the expressions of genes BSP and OCN were both up-regulated over time, and those of genes RUNX2 and COL1A1 were up-regulated from 2 to 4 days and down-regulated from 4 to 6 days. The minimum value was set to 1, and values are expressed as mean±2 SD. The bars with different letters at the same time are significantly different from each other (p<0.05; n=3), and those with similar letters show no significant difference. RUNX2, runt-related transcription factor 2; BSP, bone sialoprotein; OCN, osteocalcin; COL1A1, alpha-1 type I collagen. |
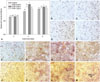 | Fig. 7Time-course of ALP activity and ALP staining of primary osteoblasts at different concentrations (0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL) of ZXHA-TC are exhibited. (A) Relative ALP activity (units/100 mL) was expressed as mean±2 SD, and the activity in 6.25×10-2 µg/mL was significantly higher than other groups. ALP activity in the ZXHA-TC-treated groups increased from 2 to 4 days and decreased slightly from 4 to 6 days. However, the activity in the control increased over time. The bars with different letters at the same time are significantly different from each other (p<0.05; n=3), and those with similar letters indicate no significant difference. (B-E) Staining of primary osteoblasts treated with ZXHA-TC at concentrations of 0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL at 2 days. (F-I) Staining of primary osteoblasts treated with ZXHA-TC at concentrations of 0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL at 4 days. (J-M) Staining of primary osteoblasts treated with ZXHA-TC at concentrations of 0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL at 6 days. ALP staining in the ZXHA-TC-treated groups was strengthened from 2 to 4 days and was weakened slightly from 4 to 6 days, and the concentration of 6.25×10-2 µg/mL performed best, which was in accordance the ALP activity results. Scale bar=200 µm. ALP, alkaline phosphatase. |
 | Fig. 8Production of intracellular ROS was detected through the intensity of fluorescence indirectly with a microplate fluorescence reader. After being treated with different concentrations of ZXHA-TC (0 µg/mL, 6.25×10-3 µg/mL, 6.25×10-2 µg/mL, and 6.25×10-1 µg/mL) for 2, 4, and 6 days, primary osteoblasts were labeled with new synthesized DNA and assayed. The level of intracellular ROS was markedly reduced by ZXHA-TC, especially at the concentration of 6.25×10-2 µg/mL. ROS, reactive oxygen species. |
Table 1
Primers for Real-Time Polymerase Chain Reaction

ACKNOWLEDGEMENTS
This work has been financially supported by the National Science & Technology Pillar Program of China (Grant No. 2012BAI42G00), the Guangxi Scientific Research and Technological Development Foundation (Grant No. Guikehe 14125008-2-14), and the Guangxi Science Fund for Distinguished Young Scholars (Grant No. 2014GXNSFGA11 8006). This work has been supported by Key Laboratory of Regenerative Medicine of Guangxi High School, Research Center for Regenerative Medicine and Collaborative Innovation Center of Guangxi Biological Medicine.
References
4. National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation;2010.
5. National Osteoporosis Foundation. Bone Health Basics: Get the Facts. accessed on 2015 March 9. Available at: http://nof.org/learn/basics.
6. National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation;2013.
7. Johnell O. Advances in osteoporosis: better identification of risk factors can reduce morbidity and mortality. J Intern Med. 1996; 239:299–304.

8. Gold DT, Solimeo S. Osteoporosis and depression: a historical perspective. Curr Osteoporos Rep. 2006; 4:134–139.

9. Pereira JV, Modesto-Filho J, Agra MF, Barbosa-Filho JM. Plant and plant-derived compounds employed in prevention of the osteoporosis. Acta Farm Bonaerense. 2002; 21:223–234.
10. Sharan K, Siddiqui JA, Swarnkar G, Maurya R, Chattopadhyay N. Role of phytochemicals in the prevention of menopausal bone loss: evidence from in vitro and in vivo, human interventional and pharma-cokinetic studies. Curr Med Chem. 2009; 16:1138–1157.

11. Arjmandi BH, Alekel L, Hollis BW, Amin D, Stacewicz-Sapuntzakis M, Guo P, et al. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J Nutr. 1996; 126:161–167.

12. Ren P, Ji H, Shao Q, Chen X, Han J, Sun Y. Protective effects of sodium daidzein sulfonate on trabecular bone in ovariectomized rats. Pharmacology. 2007; 79:129–136.

13. Kelly GE. Treatment or prevention of osteoporosis. U.S. Patent 6,340,703[P]. 2002. 01. 22.
14. Yadav DK, Gautam AK, Kureel J, Srivastava K, Sahai M, Singh D, et al. Synthetic analogs of daidzein, having more potent osteoblast stimulating effect. Bioorg Med Chem Lett. 2011; 21:677–681.

15. Kuruto-Niwa R, Inoue S, Ogawa S, Muramatsu M, Nozawa R. Effects of tea catechins on the ERE-regulated estrogenic activity. J Agric Food Chem. 2000; 48:6355–6361.

16. Shen CL, Kwun IS, Wang S, Mo H, Chen L, Jenkins M, et al. Functions and mechanisms of green tea catechins in regulating bone remodeling. Curr Drug Targets. 2013; 14:1619–1630.

17. Lu Z, Nie G, Belton PS, Tang H, Zhao B. Structure-activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem Int. 2006; 48:263–274.

18. Kroes BH, van den Berg AJ, Quarles van Ufford HC, van Dijk H, Labadie RP. Anti-inflammatory activity of gallic acid. Planta Med. 1992; 58:499–504.

19. Suntory Ltd. High level expression of proteins in yeast. Patent 6,183,985[P]. 1995. 03. 01.
20. Ou TT, Lin MC, Wu CH, Lin WL, Wang CJ. Gallic acid attenuates oleic acid-induced proliferation of vascular smooth muscle cell through regulation of AMPK-eNOS-FAS signaling. Curr Med Chem. 2013; 20:3944–3953.

21. Yoon CH, Chung SJ, Lee SW, Park YB, Lee SK, Park MC. Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Joint Bone Spine. 2013; 80:274–279.

22. Chuang CY, Liu HC, Wu LC, Chen CY, Chang JT, Hsu SL. Gallic acid induces apoptosis of lung fibroblasts via a reactive oxygen species-dependent ataxia telangiectasia mutated-p53 activation pathway. J Agric Food Chem. 2010; 58:2943–2951.

23. Nuti E, Santamaria S, Casalini F, Yamamoto K, Marinelli L, La Pietra V, et al. Arylsulfonamide inhibitors of aggrecanases as potential therapeutic agents for osteoarthritis: synthesis and biological evaluation. Eur J Med Chem. 2013; 1:379–394.

24. Saxena HO, Faridi U, Srivastava S, Kumar JK, Darokar MP, Luqman S, et al. Gallic acid-based indanone derivatives as anticancer agents. Bioorg Med Chem Lett. 2008; 18:3914–3918.

25. Kang MS, Jang HS, Oh JS, Yang KH, Choi NK, Lim HS, et al. Effects of methyl gallate and gallic acid on the production of inflammatory mediators interleukin-6 and interleukin-8 by oral epithelial cells stimulated with Fusobacterium nucleatum. J Microbiol. 2009; 47:760–767.

26. Ho HH, Chang CS, Ho WC, Liao SY, Wu CH, Wang CJ. Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-kappaB activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem Toxicol. 2010; 48:2508–2516.

27. Kuppan G, Balasubramanyam J, Monickaraj F, Srinivasan G, Mohan V, Balasubramanyam M. Transcriptional regulation of cytokines and oxidative stress by gallic acid in human THP-1 monocytes. Cytokine. 2010; 49:229–234.

28. Lo C, Lai TY, Yang JS, Yang JH, Ma YS, Weng SW, et al. Gallic acid inhibits the migration and invasion of A375.S2 human melanoma cells through the inhibition of matrix metalloproteinase-2 and Ras. Melanoma Res. 2011; 21:267–273.

29. Schroeder TM, Jensen ED, Westendorf JJ. Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today. 2005; 75:213–225.

30. Cobb J, Dierich A, Huss-Garcia Y, Duboule D. A mouse model for human short-stature syndromes identifies Shox2 as an upstream regulator of Runx2 during long-bone development. Proc Natl Acad Sci U S A. 2006; 103:4511–4515.

31. Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, et al. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol. 2001; 155:157–166.

32. Na K, Kim SW, Sun BK, Woo DG, Yang HN, Chung HM, et al. Osteogenic differentiation of rabbit mesenchymal stem cells in thermo-reversible hydrogel constructs containing hydroxyapatite and bone morphogenic protein-2 (BMP-2). Biomaterials. 2007; 28:2631–2637.

33. Stein GS, Lian JB. Molecular mechanisms mediating proliferation/ differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993; 14:424–442.

34. D'Errico JA, MacNeil RL, Takata T, Berry J, Strayhorn C, Somerman MJ. Expression of bone associated markers by tooth root lining cells, in situ and in vitro. Bone. 1997; 20:117–126.
35. Hakki SS, Wang D, Franceschi RT, Somerman MJ. Bone sialoprotein gene transfer to periodontal ligament cells may not be sufficient to promote mineralization in vitro or in vivo. J Periodontol. 2006; 77:167–173.

36. Hakki SS, Bozkurt SB, Hakki EE, Belli S. Effects of mineral trioxide aggregate on cell survival, gene expression associated with mineralized tissues, and biomineralization of cementoblasts. J Endod. 2009; 35:513–519.

37. Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011; 54:1291–1297.

38. Tielens S, Wymeersch F, Declercq H, Cornelissen M. Effect of 17beta-estradiol on the in vitro differentiation of murine embryonic stem cells into the osteogenic lineage. In Vitro Cell Dev Biol Anim. 2008; 44:368–378.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download