Abstract
It is controversial whether isolated lesions of mammillothalamic tract (MTT) produce significant amnesia. Since the MTT is small and adjacent to several important structures for memory, amnesia associated with isolated MTT infarction has been rarely reported. We report a patient who developed amnesia following an infarction of the left MTT that spared adjacent memory-related structures including the anterior thalamic nucleus. The patient's memory deficit was characterized by a severe anterograde encoding deficit and retrograde amnesia with a temporal gradient. In contrast, he did not show either frontal executive dysfunction or personality change that is frequently recognized in the anterior or medial thalamic lesion. We postulate that an amnesic syndrome can develop following discrete lesions of the MTT.
The mammillothalamic tract (MTT), connecting the mammillary body (MB) with the anterior thalamic nucleus, is a part of the Papez circuit. Therefore, disruption of the MTT would be predicted to cause a memory disturbance (1). However, memory deficits following an MTT lesion have not been clearly characterized, since MTT is a small structure, rarely being involved selectively.
Recently, a couple of case reports described patients with acute onset amnesia after either unilateral or bilateral MTT infarction (2, 3). However, a subsequent study demonstrated that memory impairment did not develop after electrode implantation in bilateral MB and MTT for refractory epilepsy. Investigators suggested that the memory impairment after MTT infarctions in previous reports could have been attributed to extension of lesions into the anterior thalamic nucleus (4).
We report a patient who showed profound and persistent global amnesia after a unilateral lesion of MTT that spared the anterior nuclear group of the thalamus.
A 70-yr-old right-handed man with nine years of education developed sudden memory loss three days before admission to Kyung Hee University Hospital. Past medical history was remarkable for hypertension for three years and two episodes of stroke. The first episode of stroke developed 11 yr earlier when he was admitted because of transient confusion. About 40 days prior to admission, he was admitted to another hospital for evaluation of dizziness. Despite the absence of focal neurological or cognitive deficits at this time, diffusion-weighted (DWI) and T2-weighted images (T2WI) showed an acute infarction in the right cerebellar hemisphere. The scan also identified an old infarct in the left lingual gyrus, as well as another slit-like lesion in the right anterior thalamus on T2WI with signal characteristics consistent with a previous thalamic hemorrhage (Fig. 1).
Three days before admission, his family suddenly noticed him asking the same questions repeatedly. On the forth day after onset (the second hospital day), the patient was alert and cooperative but recalled none of the previous day's events and failed to recognize the attending physician who had been introduced to the patient several times on the previous day. On the Mini-Mental Status Examination, he did not even recall being given three words that he had registered only a few minutes before, scoring 20/30 due to additional problems with orientation to time, place, and naming. The patient was aware of his memory loss. No abnormalities were found on the rest of the neurological examination.
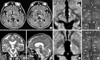
High-resolution DWIs taken four days after onset showed a new focal infarct, which proved to be localized to the left MTT according to an atlas (Fig. 2) (5). T2WI and fluid attenuated inversion recovery imaging obtained three days later showed the MTT lesion with no additional lesions except the aforementioned lesions (Fig. 1). Single photon emission computed tomography (SPECT) performed on the same day showed decreased cerebral blood flow in the right cerebellar hemisphere and the left occipital lobe, but no flow reduction in other cortical regions.
Detailed neuropsychological tests using a standardized battery called Seoul Neuropsychological Screening Battery (6) and Wechsler Adult Intelligence Scale were performed four days after onset. His verbal and performance IQ were 92 and 93 with a full-scale IQ of 92, consistent with expected premorbid function. Forward digit span was 6 (64th percentile). His performances on spoken and written language were normal as were on 10 items of praxis and on tasks for Gerstmann syndrome. However, the patient had impaired confrontation naming as assessed by the Korean version of the Boston Naming Test (29/60, 1st percentile). Copying ability tested with the Rey-Osterrieth Complex Figure was intact (30/36, 32nd percentile), but immediate recall (7.5/36, 12th percentile), 20-min delayed recall (10/36, 17th percentile), and recognition ability (true positive-false positive=3-2, <1 percentile) were impaired. Verbal memory as assessed by the Seoul Verbal Learning test was also impaired, showing defective new learning (3-trial immediate free recall: 8/36, 1st percentile), delayed recall (0/12, <1 percentile), and recognition (1/12, <1 percentile). Frontal/executive functions including go-no-go test, controlled oral word association, motor set shifting, and Stroop color test (101/112, 72nd percentile) were intact. In addition to anterograde amnesia, he also had impaired recalling of remote events. He believed the current president to be the one in office 10 yr ago, and he failed to recall 2002 FIFA WORLD CUP KOREA/JAPAN. Retrograde memory impairment was most profound for incidents during the past one year, including the deaths of two close friends, moving to a new house, and even his hospital admission 40 days before our evaluation. To further substantiate his retrograde amnesia, we performed the autobiographical memory interview, which showed that his retrograde amnesia was limited almost exclusively to the events during the past one year.
A repeat evaluation using the same neuropsychological tests four months after onset showed persistent amnesia. He could not even remember his mother's death two months previously.
Despite recurrent episodes of stroke, our patient had experienced no previous cognitive changes except for a transient episode of mental confusion until being admitted. Therefore, although the patient had lesions in the left lingual gyrus, the right cerebellar hemisphere, and the right anterior thalamus, it is difficult to explain the patient's acute profound amnesia by these lesions. Rather, the acute infarction in the left MTT confirmed by DWI have caused severe amnesia by disconnecting the MB and the anterior thalamic nucleus.
To date, there have been two case reports that specifically documented memory dysfunction following MTT infarction. Yoneoka and colleagues recently reported a patient with Korsakoff syndrome associated with ischemic injury in the left MTT (3). However, this case may differ from our case in that the lesion of the patient involved left anterior thalamus in addition to MTT, while the lesion in our patient selectively damaged MTT. This involvement of the anterior thalamus may be associated with the patient's behavioral and cognitive deficits in addition to amnesia; that is, although details of cognitive assessment were not provided, the authors described their patient as disoriented in time and demonstrating both anterograde and retrograde amnesia. In addition, he exhibited minimal insight into cognitive dysfunction, confabulated and exhibited poor concentration. It has been reported that these neuropsychological and neurobehavioral abnormalities are frequently observed from an anterior thalamic lesion (7).
In contrast, Schott and associates reported a patient with amnesic syndrome due to an unequivocally isolated left MTT infarct (2). Detailed testing showed selective episodic memory impairment limited to verbal material with preservation of visual memory. Our patient with a left MTT lesion, however, showed both verbal and visual memory loss. It has been known that verbal memory loss is more often associated with disruption of memory circuits in the left (dominant) than in the right hemisphere, whereas the opposite is true with visual memory loss (2). Thus, the memory loss in our patient, is not compatible with this hypothesis, although the hemisphere-specific memory loss in thalamic injury is reported to be inconsistent (8). The visual memory loss in our patient may be attributed to the old right thalamic lesion. In the same context, we cannot entirely exclude the possibility that our patient's subclinical lesion involving the right thalamus might have contributed to the emergence of amnesia after the left MTT infarction. As in our case, the patient described by Schott et al. did not show confabulation and retained insight of cognitive dysfunction. Dysnomia was present, albeit not being as severe as in our patient. Results of executive tests were not mentioned. Retrograde memory difficulty, tested using a famous faces test, was established for name recall but not face recognition.
A recent study asserted that amnesia attributed to MTT injury may result from extension of lesions into adjacent structures (4). Indeed, the MTT is a minute fiber bundle coursing in close proximity to other memory-related structures such as the fornix, the ventral amygdalofugal pathway, the anterior and mediodorsal thalamic nuclei, and the internal medullary lamina. Furthermore, the MTT shares a common vascular supply with these structures via the tuberothalamic artery. Thus, even small ischemic lesions of the thalamus can variably involve multiple nuclei and their projections, resulting in heterogeneous patterns of diencephalic amnesia and accompanying neuropsychological deficits. As a result, understanding the contribution of MTT lesions to amnesia has remained elusive.
Such patients as those described in this report and by Schott et al. (2) provide the evidence of a critical role for the MTT in mediating episodic memory. Firstly, images from these two patients showed minimal dorsal and rostral extension, making injury to the anterior and the medial dorsal nuclei less likely. Secondly behavioral observations from the two cases indicate no executive dysfunction or confabulation often associated with the injury to more anterior and dorsal thalamic nuclei (9). Finally, radionuclide imaging in our case failed to detect reduced cerebral blood flow in the thalamus or the cortical projection zones of the anterior and the medial dorsal thalamic nuclei. Prior brain SPECT demonstrated reduced blood flow in association with cortical regions following pathological disconnection from more rostral and dorsal thalamic structures (10). Thus, findings from these two case studies are in line with a recent magnetic resonance imaging-based lesion overlap study of thalamic infarctions, which found that MTT involvement correlates highly with severe memory deficits (1).
To our knowledge, few patients with an isolated MTT injury underwent detailed neuropsychological tests including frontal functions. Other than anomia, the only neuropsychological deficit in our patient was memory dysfunction characterized by severe anterograde and retrograde amnesia with a temporal gradient. Markedly low scores in both delayed recall and recognition suggest that anterograde amnesia was associated with encoding and storage problems rather than retrieval deficit. These findings suggest that the features of amnesia from MTT damage resemble those associated with medial temporal damage, but differ from those following anterior thalamic lesions (7).
Figures and Tables
Fig. 1
Diffusion-weighted (A) and T2-weighted (B) images from the previous stroke (40 days prior to admission) show acute infarction of the right cerebellar hemisphere, an old infarct in the left lingual gyrus, and a slit-like suspicious old hemorrhage in the right anterior thalamus.
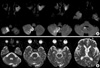
Fig. 2
Axial FLAIR (A), coronal (B), and sagittal (C) T2-weighted images from the current amnesic stroke show a new infarction in the left thalamic area (arrowhead) in addition to the old left lingual gyrus and right anterior thalamic (arrow) lesions. Axial diffusion- weighted images (D, E) confirmed acuteness of the infarction that is localized to the mammillothalamic tract according to an anatomic atlas (5) (F, G). ac, anterior commissure; cc, crus cerebri; fx, fornix; h, hypothalamus; mt, mammillothalamic tract; na, nucleus accumbens; pc, posterior commissure; pu, pulvinar; r, red nucleus; sc, superior colliculus; sn, substantia nigra; vpl, ventral posterolateral thalamic nucleus; 22, third ventricle.
References
1. Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HB, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003. 41:1330–1344.

2. Schott JM, Crutch SJ, Fox NC, Warrington EK. Development of selective verbal memory impairment secondary to a left thalamic infarct: a longitudinal case study. J Neurol Neurosurg Psychiatry. 2003. 74:255–257.

3. Yoneoka Y, Takeda N, Inoue A, Ibuchi Y, Kumagai T, Sugai T, Takeda K, Ueda K. Acute Korsakoff syndrome following mammillothalamic tract infarction. AJNR Am J Neuroradiol. 2004. 25:964–968.
4. Duprez TP, Serieh BA, Raftopoulos C. Absence of memory dysfunction after bilateral mammillary body and mammillothalamic tract electrode implantation: preliminary experience in three patients. AJNR Am J Neuroradiol. 2005. 26:195–197.
5. Duvernoy HM. The Human Brain: Surface, three-dimensional sectional anatomy with MRI, and blood supply. 1999. 2nd ed. Wien: Springer-Verlag.
6. Kang Y, Na DL. Seoul Neuropsychological Screening Battery. 2003. Human Brain Research & Consulting Co.
7. Perren F, Clarke S, Bogousslavsky J. The syndrome of combined polar and paramedian thalamic infarction. Arch Neurol. 2005. 62:1212–1216.

8. Exner C, Weniger G, Irle E. Implicit and explicit memory after focal thalamic lesions. Neurology. 2001. 57:2054–2063.

9. Pepin EP, Auray-Pepin L. Selective dorsolateral frontal lobe dysfunction associated with diencephalic amnesia. Neurology. 1993. 43:733–741.

10. Hayashi R, Ohashi M, Watanabe R, Mimura M, Katsumata Y. Amnesia, confabulation and nonaphasic misnaming after left thalamic infract. No To Shinkei. 2003. 55:530–535.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download