Abstract
Waldenstrom's macroglobulinemia is an uncommon low-grade B-cell lymphoproliferative disorder in which monoclonal immunoglobulin M is produced. Neurological symptoms due to hyperviscosity are frequent manifestations of Waldenstrom's macroglobulinemia. However, central nervous system infiltration by plasmacytoid lymphocytes (Bing-Neel syndrome) has only rarely been reported. We report a case of a 51-yr-old woman suffering from Waldenstrom's macroglobulinemia who complained of persistant headache. Brain magnetic resonance imaging revealed an extra-axial soft tissue mass along the left cavernous sinus, left tentorium, right tentorium, and falx cerebri. A stereotactic biopsy of dural tissue from the falx was performed and showed plasmacytoid lymphocyte infiltration. The patient became symptom- free with irradiation of the whole brain followed by chemotherapy with fludarabine.
Waldenstrom's macroglobulinemia (WM) is the result of a clonal proliferation of lymphocytes that produce monoclonal immunoglobulin M (IgM). This disease was originally described in 1944 by Jan Waldenstrom. It is now considered to correspond to lymphoplasmacytoid lymphoma as defined by the World Health Organization classification system (1, 2). Its clinical features include hyperviscosity, cytopenia, bleeding, lymphadenopathy, and/or splenomegaly. Many central nervous system (CNS) complications have been described in WM patients; the majority have been associated with blood hyperviscosity caused by IgM. The hyperviscosity syndrome is characterized by headache, tinnitus, vertigo, blurred vision, and chronic bleeding from the nose and gums (3). However, CNS infiltration by plasmacytoid lymphocytes (Bing-Neel syndrome) has only rarely been reported (4).
A 51-yr-old woman was diagnosed with WM three years prior to this presentation. She received six courses of chlorambucil (0.3 mg/kg per day on day one to four orally) and prednisone (45 mg/m2 per day on day one to four). However, the disease progressed. Next, she received fludarabine chemotherapy (25 mg/m2 per day on day one to five intravenously) with a limited response. High-dose cyclophosphamide and granulocyte colony-stimulating factor were administered to induce peripheral blood progenitor cell (PBPCs) mobilization for autologous stem-cell transplantation; however, the number of PBPCs collected was not sufficient. We then decided on a conservative treatment approach, because the patient did not have specific symptoms and the serum IgM level was stable (3,000-3,500 mg/dL). She was admitted to the hospital because of persistent headache and increased IgM levels. Physical examination was unremarkable. The laboratory values on admission were as follows: white cell count 8,050/µL with normal differential counts, hemoglobin 9.6 g/dL, platelets 296,000/µL, erythrocyte sedimentation rate 144 mm/hr, total protein 9.44 g/dL, albumin 2.85 g/dL, IgG 3144.4 mg/dL, IgA 25.4 mg/dL, IgM 4904.6 mg/dL, and serum β2-microglobulin 3.81 mg/L. A brain computed tomography scan revealed multifocal extra-axial tumorous lesions along the dura matter. A brain magetic resonance imaging showed an extra-axial soft tissue tumor along the left cavernous sinus and tentorium, right frontal convexity and tentorium and falx; the brain parenchyma appeared to be unremarkable (Fig. 1A). Cerebrospinal fluid analysis showed the following: white cell count 43/µL with neutrophils 1%, lymphocytes 52% and monocytes 47%, total protein 1.81 g/dL, glucose 104 mg/dL, IgM 64.5 mg/dL and a few plasmacytoid lymphocytes on cytology. Stereotactic biopsy of dural tissue at the falx showed a diffuse infiltration with atypical cells, which were identified immunophenotypically as plasmacytoid lymphocyte with expression of LCA (+), CD3 (-), CD20 (+), and VS38a (+) (Fig. 2). The patient was confirmed to have CNS infiltration by atypical plasmacytoid lymphocyte infiltration (Bing-Neel syndrome). The paitent received a total dose of 1,980 cGy with irradiation therapy of the whole brain that was administered in 11 fractions, and then her headache subsided, but the IgM level was elevated persistently to 3,379.8 mg/dL. Following radiation therapy, fludarabine chemotherapy was performed (25 mg/m2 per day on 1 to 5 intravenously, two courses). Further treatment was not possible because of persistant bone marrow suppression. A follow-up brain MRI after six months revealed a marked decrease in the size of the mass in the tentorium and falx (Fig. 1B). The patient had no evidence of CNS recurrence during the follow-up period of one year. However, the IgM level has been increasing slowly, so further chemotherapy, including rituximab may be considered.
In 1936 Bing and Neel reported the association of hyperglobulinemia, CNS symptoms (paresthesias, headache, and paralysis), and brain infiltration composed of plasma cells and lymphocytes in two patients (5). In 1944, Waldenstrom described the syndrome that bears their name (Bing-Neel syndrome) (6). The Bing-Neel syndrome, originally placed in a "toxic-infectious" category, appears to be the result of involvement of the CNS by diffuse neoplasm infiltration. Neurological complications occur in about 25% of patients with WM. Although they are most often peripheral, they can involve the CNS. In WM, the CNS may be involved by a variety of mechanisms, including hyperviscosity and direct infiltration by neoplastic cells (7, 8). Patients with Bing-Neel syndrome have sometimes presented with a mass containing neoplastic cells, but the masses have been intraparenchymal rather than meningeal (8, 9). This syndrome can be subdivided into diffuse and tumoral forms. In the diffuse infiltrative form such as this case, malignant cells are localized mainly in leptomeningeal spaces, periventricular white-matter, pons, and medulla (7, 10-12). Radiological findings in this syndrome have been reported, but a typical pattern has not emerged (13-15). An imaging technique would be the preferable diagnostic test, although histological confirmation is necessary to establish the definitive diagnosis.
Intraventricular chemotherapy for Bing-Neel syndrome was reported to be effective in 1984 (15). However, a review of the literature reveals that the outcome for most patients who underwent chemotherapy was poor, and the patients died within several months (10, 13, 14). Therefore, patients with Bing-Neel syndrome may benefit from cranial radiation therapy prior to chemotherapy (9, 12, 16). Recently, a great deal of interest has been noted by treatments with purine nucleoside analogs (fludarabine, cladribine, and pentostatin) because of their remarkable activity in lymphoproliferative disorders. It has been reported that a patient with Bing-Neel syndrome (in its diffuse form) was successfully treated with cladribine administration (17), or radiation therapy and combination of cladribine, cyclophosphamide, and prednisone (18). In the present case, headache subsided after radiation therapy, but the IgM level increased persistently. Therefore, following radiation therapy we used fludarabine for two courses. Further treatment was not possible because of persistant bone marrow suppression. A follow-up brain MRI after six months revealed a marked decrease in the size of the mass in the tentorium and falx; the patient had no evidence of CNS recurrence during the follow-up period of one year, but the IgM level has been increasing slowly. We think that the major effect of deceased mass size was due to the radiation therapy in the present case.
Several retrospective and prospective studies have indicated that rituximab may induce an objective response in approximately 30-40% of previous treated patients with WM (19). However, the effect of rituximab treatment on the cerebrospinal fluid B-cell compartment is limited in comparison with the effect on the B cells in the periphery (20), and it has not been tried in Bing-Neel syndrome as yet. Therefore, the effect of rituximab on CNS involovement of WM need to be validated by of future studies.
Figures and Tables
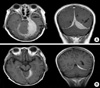
Fig. 1
(A) Contrast enhanced axial and coronal T1-weighted images show a well-enhanced mass along the left tentorium and cavernous sinus (arrows). This mass also extends into the contralateral tentorium and falx (arrowheads). (B) Marked decrease in the size of the mass in the tentorium and falx after radiation therapy and fludarabine treatment.
References
1. Waldenstrom J. Incipient myelomatosis or "essential" hyperglobulinemia with fibrinogenopenia-a new syndrome? Acta Med Scand. 1944. 117:216–222.
2. Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol. 1999. 10:1419–1432.
4. Civit T, Coulbois S, Baylac F, Auque J. Waldenstrom's macroglobulinemia and cerebral lymphoplasmocytic proliferation: Bing and Neel syndrome. Apropos of a new case. Neurochirurgie. 1997. 43:245–249.
5. Bing J, Neel A. Two case of hyperglobulinemia with affection of the central nervous system on a toxi-infection basis. Acta Med Scand. 1936. 88:492–506.
6. Waldenström J. Incipient myelomatosis or "essential" hyperglobulinemia with fibrinogenopenia-a new syndrome? Acta Me Scand. 1944. 117:216–247.
7. Scheithauer BW, Rubinstein LJ, Herman MM. Leukoencephalopathy in Waldenstrom's macroglobulinemia: immunohistochemical and electron microscopic observations. J Neuropathol Exp Neurol. 1984. 43:408–425.
8. Edgar R, Dutcher TF. Histopathology of the Bing-Neel syndrome. Neurology. 1961. 11:239–245.
9. Imai F, Fujisawa K, Kiya N, Ninomiya T, Ogura Y, Mizoguchi Y, Sano H, Kanno T. Intracerebral infiltration by monoclonal plasmacytoid cells in Waldenstrom's macroglobulinemia-case report. Neurol Med Chir (Tokyo). 1995. 35:575–579.
10. Logothetis J, Silverstein P, Coe J. Neurologic aspects of Waldernstrom's macroglobulinemia: report of a case. Arch Neurol. 1960. 3:564–573.
11. Tommasi M, Revol L, Schott B, Lesbros F. Waldenstrom's macroglobulinemia. Lesions of the central nervous system. Ann Anat Pathol (Paris). 1966. 11:309–313.
12. Case Records of the Massachussetts General Hospital (Case 8-2001). N Engl J Med. 2001. 344:832–839.
13. Leger JM, Younes-chennoufi AB, Zuber M, Bouche P, Jauberteau MO, Dormont D, Danon F, Baumann N, Brunet P. Frequency of central lesions in polyneuropathy associated with IgM monoclonal gammopathy: an MRI, Neurophysiological and immunochemical study. J Neurol Neurosurg Psychiatry. 1992. 55:112–115.

14. Neau JP, Guilhot F, Dumas P, Besson I, Rivasseau-Jonveaux T, Gil R. Central nervous system involvement in Waldenstrom's disease. Bing-Neel syndrome. 3 cases. Rev Neurol (Paris). 1991. 147:56–60.
15. Torrey JJ, Katakkar SB. Treatable meningeal involvement in Waldenstrom's macroglobulinemia. Ann Intern Med. 1984. 101:345–347.

17. Richards AI. Response of meningeal Waldenstrom's macroglobulinemia to 2-chlorodeoxyadnosine. J Clin Oncol. 1995. 13:2476.
18. Delgado J, Canales MA, Garcia B, Alvarez-Ferreira J, Garcia-Grande A, Hernandez-Navarro F. Radiation therapy and combination of cladribine, cyclophosphamide, and prednisone as treatment of Bing-Neel syndrome: case report and review of the literature. Am J Hematol. 2002. 69:127–131.

19. Dimopoulos MA, Zervas C, Zomas A, Kiamouris C, Viniou NA, Grigoraki V, Karkantaris C, Mitsouli C, Gika D, Christakis J, Anagnostopoulos N. Treatment of Waldenstrom's macroglobulinemia with rituximab. J Clin Oncol. 2002. 20:2327–2333.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download