Abstract
This study was done to determine whether recombinant human erythropoietin (rhEPO) treatment could attenuate hyperoxia-induced lung injury, and if so, whether this protective effect is mediated by the down-modulation of inflammation in neonatal rats. Newborn Sprague Dawley rat pups were subjected to 14 days of hyperoxia (>95% oxygen) within 10 hr after birth. Treatment with rhEPO significantly attenuated the mortality and reduced body weight gain caused by hyperoxia. With rhEPO treatment, given 3 unit/gm intraperitoneally at 4th, 5th, and 6th postnatal day, hyperoxia-induced alterations in lung pathology such as decreased radial alveolar count, increased mean linear intercept, and fibrosis were significantly improved, and the inflammatory changes such as myeloperoxidase activity and tumor necrosis factor-alpha expression were also significantly attenuated. In summary, rhEPO treatment significantly attenuated hyperoxia-induced lung injury by down-modulating the inflammatory responses in neonatal rats.
Despite recent improvements in neonatal respiratory management, bronchopulmonary dysplasia (BPD) continues to represent a major cause of mortality and morbidity among premature infants (1). Histopathologic characteristics of BPD include airway injury, inflammation, and parenchymal fibrosis (2-4). Although the pathogenesis of BPD is incompletely understood, inflammation is believed to play a central role in the lung injury process leading to the development of BPD (5). Neutrophils (5-7) and proinflammatory cytokines (8-10) have been known to exacerbate the inflammatory process of chronic lung disease in premature infants. Therefore, the investigation on the effectiveness of new therapeutic modalities such as anti-inflammatory agents in the treatment of BPD is of great interest.
Erythropoietin (EPO), a 30.4-kD glycoprotein that regulates the rate of red blood cell production, has been used for many years to treat anemia of prematurity (11, 12). In a recent animal study (13), treatment of hyperoxia-exposed newborn rat pups with recombinant human EPO (rhEPO) significantly improved alveolar structure, enhanced vascularity, and lessened fibrosis. These findings suggest that treatment of premature infants with EPO might reduce the risk of developing BPD. However, a better understanding of the action mechanism of EPO must be obtained for translation of this experimental result to clinical trials in BPD patients.
Although the precise action mechanism of EPO has not been elucidated yet, one mechanism that may contribute greatly to the cytoprotection during BPD is anti-inflammation. Anti-inflammatory effects of EPO such as inhibition of leukocyte infiltration and decreased production of proinflammatory cytokines have been observed in various in vivo studies (14-16). In the present study, we tried to determine whether recombinant human EPO treatment could attenuate hyperoxia-induced lung injury, and if so, whether this protective effect is mediated by the down-modulating the inflammatory responses in neonatal rats.
The experimental protocols described herein were reviewed and approved by the Animal Care and Use Committee of the Samsung Biomedical Research Institute, Seoul, Korea. This study also followed the institutional and National Institutes of Health guidelines for laboratory animal care. Timed pregnant Sprague Dawley rats were housed in individual cages with free access to water and laboratory chow, and the rat pups were delivered spontaneously. The experiment began within 10 hr after birth and continued through postnatal d 14. Ninety rat pups were divided into four groups: normoxia control group (NC, n=8); normoxia with EPO treatment group (NE, n=8); hyperoxia control group (HC, n=37); and hyperoxia with EPO treatment group (HE, n=37). Throughout the experiment, rat pups of NC and NE were kept in the standard cage with nursing mother rat at room air, and pups of HC and HE were kept in the standard cage with mother rat within 50 liter Plexiglas chamber in which hyperoxia (>95% oxygen concentration) was maintained. Humidity was maintained at 50%, and environmental temperature was maintained at 24℃. To avoid oxygen toxicity of nursing mother rat, mother rats were rotated daily between litters in normoxia groups and hyperoxia groups. Pups in NE and HE were injected with 3 unit/gm of rhEPO (Epokine prefilled: Erythropoietin alpha, CJ Corp., Seoul, Korea) intraperitoneally at 4th, 5th, and 6th postnatal day. Pups in NC and HC received normal saline administered in the same way. Survival and body weight of rat pups in each group were checked daily throughout the experiment, and sacrificed at postnatal d 14 after intraperitoneal injection of ketamine (Yuhan corp., Seoul, Korea, 50 mg/kg).
The lungs were resected after perfusion of the heart with normal saline. Right lung was saved for later biochemical analyses. Left lung was fixed in situ at a constant inflation of 20 cmH2O pressure, and then fixed overnight in 10% buffered formalin. The fixed lung tissue was embedded in paraffin wax after tissue processing. Five-micrometer thick sections were cut from the paraffin blocks, and stained with hematoxylin and eosin. Images of each section were captured with a Magnafire digital camera through an Olympus BX40 microscope (Olympus optical co. Ltd., Tokyo, Japan) and were saved JPEG files. Analysis of each section was carried out in a blind fashion by a single observer.
Alveolarization was assessed by performing radial alveolar count (RAC), according to the method of Emery and Mithal (17). From the center of the respiratory bronchiole, a perpendicular line was drawn to the edge of the acinus (as defined by a connective tissue septum or the pleura), and the number of septa intersected by this line was counted.
The inter-alveolar distance was assessed by measuring mean linear intercept (MLI), obtained by dividing the total length of lines drawn across the lung section by the number of intercepts encountered, as described by Cooney and Thurlbeck (18).
The activity of myeloperoxidase (MPO), an indicator of neutrophil accumulation, was determined by a modification of the method by Gray et al. (20). The lung tissues were homogenized in a phosphate buffer (pH 7.4) and centrifuged at 30,000 g for 30 min. The pellet was resuspended in another phosphate buffer (50 mM, pH 6.0) with 0.5% hexadecyltrimethyl ammonium bromide. MPO activity in the resuspended pellet was assayed by measuring absorbance changes spectrophotometrically at 460 nm, using 0.167 mg/mL O-dianisidine hydrochloride and 0.0005% hydrogen peroxide. One unit of MPO activity was defined as the quantity of enzyme degrading 1 µM of peroxide/min,
Total RNA was isolated from lung tissues using the Trizol reagent (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer's protocol. First-strand complementary DNA (cDNA) was synthesized using random primers and the AMV reverse transcriptase (Promega Corp., Madison, WI, U.S.A.). RT-generated cDNA encoding TNF-a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified using PCR. The housekeeping gene GAPDH was used as an internal standard. The primers used were for rat-specific TNF-α and GAPDH. PCR reactions were initiated at 94℃ for 3 min, followed by 35 cycles of amplication (denaturation at 94℃ for 1 min, annealing at 55℃ for 1 min, and extension at 72℃ for 1 min) with a final primer extension at 72℃ for 5 min. The PCR products were separated on 1.5% agarose gel and stained with ethidium bromide. The intensity of each mRNA band was quantified by densitometry using a gel documentation and analysis system and normalized to values for GAPDH.
All data were presented as the mean±standard deviation. Statistical comparison between each group was performed by one-way analysis of variance with Bonferroni's correction. For comparison of survival curves, Kaplan-Meiyer analysis was performed. A p value of <0.05 was considered significant.
Exposure to oxygen (HC) significantly decreased the survival rate at the end of experiment (postnatal d 14) compared to normoxia groups (NC and NE) (27% vs. 88%, p<0.05), and this hyperoxia-induced decreased survival rate was increased to 41% with EPO treatment (HE) (Fig. 2).
Although birth weight was not different between the four groups, pups nursed in hyperoxia (HC) showed a significantly reduced body weight gain compared to normoxia groups (NC, NE) both at postnatal d 7 and 14, and this hyperoxia-induced decrease in body weight was increased at postnatal d 14 with EPO treatment (HE) (Table 1).
In morphometric analyses, significantly decreased RAC and increased MLI were observed in HC compared to normoxia groups (NC and NE) (Fig. 4A, B). These hyperoxia-induced morphometric abnormalities were significantly improved with rhEPO treatment (HE).
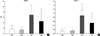
The significantly increased MPO activity and TNF-α expression observed in HC compared to normoxia groups (NC and NE) were significantly improved with rhEPO treatment (HE) (Fig. 5).
The development of an appropriate animal model that can simulate the clinical BPD of the premature infants is difficult. In the present study, prolonged exposure of newborn rat pups to hyperoxia developed lung injuries similar to that seen in human infants with BPD (2), exhibiting decreased alveolarization as evidenced by decreased RAC and increased MLI (3) and markedly increased fibrosis (4). Furthermore, the saccular stage of the newborn rat pup is compatible to the lung development of premature infants at 25 weeks of gestation. Hence the newborn rat pup model is a very convenient model for studying various aspects of BPD.
Although the precise mechanisms of the hyperoxia-induced lung injury are unclear, inflammation is believed to play a central role in the pathogenesis of BPD (5). The number of neutrophils was persistently increased both in the interstitium and in the airspaces of the lungs of infants with BPD (6), and inhibition of neutrophil influx into the hypreoxic lung enhanced postnatal lung growth (7). MPO is an enzyme contained in and specific for neutrophil lysosomes (14). Therefore, our data of increased MPO activity observed in the hyperoxia-induced lung also support the assumption that neutrophil influx into the lung would play a pivotal role in the development of BPD (5-7).
Elevation of TNF-α represents one of the first pulmonary responses to hyperoxia (8), and pretreatment with anti-TNF-α antibody significantly attenuated the hyperoxia-induced lung injury (9). In the present study, TNF-α RNA expression was significantly increased in hyperoxia-induced lungs. TNF-α can also enhance the neutrophil-dependent increase in endothelial permeability (10), and stimulate the release of other pro-inflammatory cytokines and oxygen-free radicals from a variety of cells (8). Taken together, these findings suggest that hyperoxia-induced inflammatory responses leading to irreversible lung damage and fibrosis are mainly mediated by neutrophils and pro-inflammatory cytokines.
In the present study, rhEPO treatment significantly improved hyperoxia-induced lung damage such as decreased alveolarization and increased fibrosis. The protective effects of EPO observed in this and other (13) animal studies suggest that treatment of premature infants with EPO might reduce the risk of developing BPD. However, a better understanding of the action mechanism of EPO must be obtained for translation of this experimental result to clinical trials in BPD patients.
The precise mechanisms by which EPO attenuates hyperoxia-induced lung injury are not completely understood. It has already been shown that inflammation plays a pivotal role in the development of hyperoxic lung injury (5-10). Therefore, one possible mechanism that might contribute greatly to the prevention of BPD by EPO is anti-inflammation (14-16). Potent anti-inflammatory activity of EPO including inhibition of leukocyte infiltration and reduced levels of proinflammatory cytokines has been reported in various in vivo studies such as zymosan-induced nonseptic shock (14), cerebral ischemia (15), and experimental autoimmune encephalomyelitis (16). In the present study, the anti-inflammatory effects of EPO such as attenuation of hyperoxia-induced increase in MPO activity, a marker of neutrophil infiltration, and reduced TNF-α expression have also been observed. Furthermore, as pulmonary inflammation is a key feature in the pathogenesis of fibrosis in BPD, significant attenuation of hyperoxia-induced fibrosis with EPO treatment observed in this and other (13) animal study suggests that anti-inflammatory activity of EPO might be responsible for this protection.
In summary, rhEPO treatment significantly attenuated hyperoxia-induced lung pathology such as decreased alveolization and increased fibrosis by down-modulating the inflammatory responses in neonatal rats. These findings support the potential use of EPO as a therapeutic agent in the prevention of BPD. Further studies regarding dosage and safety will be necessary for the translation of the benefits of EPO treatment observed in this study into clinical trials.
Figures and Tables
Fig. 1
Representative photomicrographs showing Grade 1 (A), Grade 3 (B), Grade 5 (C), and Grade 7 (D) fibrosis, respectively (H&E, magnification, ×40).

Fig. 2
Survival rate in each experimental group. NC, Normoxia control group; NE, Normoxia with erythropoietin treatment group; HC, Hyperoxia control group; HE, Hyperoxia with erythropoietin treatment group. *p value <0.05 compared to NC; †p value <0.05 compared to NE; ‡p value <0.05 compared to HC.

Fig. 3
Effect of eythropoietin treatment on lung histology after hyperoxia in newborn rats. (H&E, magnification, ×40). (A) Lung structure of neonatal rats in normoxia at d 14 (NC). (B) Erythropoietin treatment has no apparent affect on the lung structure of rats maintained in normoxia (NE). (C) As shown, hypeoxia decreases lung septation and causes lung septation and causes distal air space enlargement (HC). (D) Erythropoietin treatment during hyperoxia increases alveolarization (HE).

Fig. 4
Histopathologic analysis in each experimental group. (A) Radial alveolar count (RAC), (B) Mean linear interscept (MLI), (C) Fibrosis score. *p value <0.05 compared to normoxia control group (NC); †p value <0.05 compared to normoxia with EPO group (NE); ‡p value <0.05 compared to hyperoxia control group (HC).
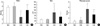
Fig. 5
Myeloperoxidase activity (A) and tumor necrosis factor-a expression (B) in each experimental group. *p value <0.05 compared to Normoxia control group (NC); †p value <0.05 compared to Normoxia with EPO group (NE); ‡p value <0.05 compared to hyperoxia control group (HC).
References
1. Jobe AH, Ikegami M. Prevention of bronchopulmonary dysplasia. Curr Opin Pediatr. 2001. 13:124–129.

2. Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967. 276:357–368.
3. Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2005. 289:L529–L535.

4. Ozer EA, Kumral A, Ozer E, Duman N, Yilmaz O, Ozkal S, Ozkan H. Effect of retinoic acid on oxygen-induced lung injury in the newborn rat. Pediatr Pulmonol. 2005. 39:35–40.

5. Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev. 1998. 53:81–94.

6. Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, Edwards DK 3rd, Gluck L. Elastase and alpha 1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. Role of inflammation in the pathogenesis of bronchopulmonary dysplasia. J Clin Invest. 1983. 72:656–666.

7. Yi M, Jankov RP, Belcastro R, Humes D, Copland I, Shek S, Sweezey NB, Post M, Albertine KH, Auten RL, Tanswell AK. Opposing effects of 60% oxygen and neutrophil influx on alveologenesis in the neonatal rat. Am J Respir Crit Care Med. 2004. 170:1188–1196.

8. Lindsay L, Oliver SJ, Freeman SL, Josien R, Krauss A, Kaplan G. Modulation of hyperoxia-induced TNF-alpha expression in the newborn rat lung by thalidomide and dexamethasone. Inflammation. 2000. 24:347–356.
9. Wherry JC, Pennington JE, Wenzel RP. Tumor necrosis factor and the therapeutic potential of anti-tumor necrosis factor antibodies. Crit Care Med. 1993. 21:Suppl 10. S436–S440.

10. Gibbs LS, Lai L, Malik AB. Tumor necrosis factor enhances the neutrophil-dependent increase in endothelial permeability. J Cell Physiol. 1990. 145:496–500.

11. Juul SE. Nonerythropoietic roles of erythropoietin in the fetus and neonate. Clin Perinatol. 2000. 27:527–541.

13. Ozer EA, Kumral A, Ozer E, Yilmaz O, Duman N, Ozkal S, Koroglu T, Ozkan H. Effects of erythropoietin on hyperoxic lung injury in neonatal rats. Pediatr Res. 2005. 58:38–41.

14. Cuzzocrea S, Di Paola R, Mazzon E, Patel NS, Genovese T, Muia C, Crisafulli C, Caputi AP, Thiemermann C. Erythropoietin reduces the development of nonseptic shock induced by zymosan in mice. Crit Care Med. 2006. 34:1168–1177.

15. Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR, Brines M, Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003. 198:971–975.

16. Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML, Ghezzi P. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002. 952:128–134.

17. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1--postnatal lung growth. Thorax. 1982. 37:572–579.

18. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 2--intrauterine and early postnatal lung growth. Thorax. 1982. 37:580–583.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download