Abstract
Although animal models with ovalbumin have been used to study chronic asthma, there are difficulties in inducing recurrence as well as in maintaining chronic inflammation in this system. Using a murine model of house dust mite (HDM)-induced bronchial asthma, we examined the airway remodeling process in response to the chronic exposure to HDM. During the seventh and twelfth weeks of study, HDM were inhaled through the nose for three consecutive days and airway responsiveness was measured. Twenty-four hours later, bronchoalveolar lavage and histological examination were performed. The degree of overproduction of mucus, subepithelial fibrosis, and the thickness of the peribronchial smooth muscle in the experimental group was clearly increased compared to the control group. In addition, HDM-exposed mice demonstrated severe airway hyperreactivity to methacholine. In the bronchoalveolar lavage fluid, the number of total cells and eosinophils was increased; during the twelfth week, the number of neutrophils increased in the experimental group. With regard to changes in cytokines, the concentrations of IL-4, IL-13, and transforming growth factor-beta (TGF-β) were increased in the experimental group. The data suggest that eosinophils, IL-4, IL-13, and TGF-β might play an important role in the airway remodeling process and that neutrophils may be involved with increased exposure time.
Bronchial asthma is a chronic inflammatory disease of the airway (1, 2). Chronic exposure to allergens triggers a distinct array of immunobiological and biochemical reactions that directly stimulate and induce abnormalities of airway structure resulting in the development of clinical symptoms (3, 4). The airway remodeling that occurs during the initiation of tissue repair in response to continuous allergic inflammation in the airway consists of many different factors. These include increase of goblet cells, hyperplasia of mucous glands, thickening of the basement membrane due to the deposition of collagen I, III, V, fibronectin, and tenacin, alteration of the composition of the extracellular matrix (ECM), and hyperplasia and hypertrophy of smooth muscle fibers (5, 6).
Airway remodeling may begin at the initial stages of bronchial asthma, which may facilitate the continuation of tissue damage and the inflammatory response by altering the composition of ECM in association with primarily eosinophils and transforming growth factor-beta (TGF-β) released by fibroblasts and other growth factors. Consequently, the thickening of the airway wall and the smooth muscle layer, and the alteration of the composition of the adventitial matrix are closely associated with the primary characteristic of asthma, airway hyperreactivity (7, 8). Recent evidence showed that in histological examination of the airway tissues of severe or near-fatal asthma, if subendothelial fibrosis was severe, the infiltration of neutrophils was increased (9, 10). Since neutrophils produce TGF-β and matrix metalloproteinase (MMP)-9, they are likely to be involved in chronic inflammation, wound repair, and the remodeling process associated with asthma (11). However, although airway remodeling is clinically important, the induction mechanism responsible for airway remodeling, in relation to the airway epithelial cells, inflammatory cells, and cytokines has not yet been elucidated. For research on airway remodeling, the development of a murine model would be extremely helpful. However, since animal models treated with ovalbumin have been used in most studies, there have been difficulties in the evaluation of recurrence and maintenance of chronic inflammation (12, 13). It was speculated that by using the most common allergen, house dust mite (HDM), repeated chronic exposure may induce chronic inflammation rather than tolerance and thus airway remodeling may develop (14).
Here, in an HDM-induced bronchial asthma murine model, we examined the airway remodeling process in response to the chronic exposure to allergens. We analyzed the alteration of inflammatory cells in the airway, as well as changes in inflammatory cells and cytokines in the bronchoalveolar lavage, and the pattern as well as the degree of airway remodeling.
Sixty BALB/c female mice, 4-6 weeks of age, were used. Animals were raised in the animal facility at the clinical research center of Catholic University Medical School. They were maintained and used according to institutional regulations developed by the institutional review board of the clinical research center, Catholic University Medical School.
The control group consisted of animals (n=30) treated with 50 µL phosphate-buffered saline (PBS) inhaled through the nose without immunization. The complete Freund's adjuvant group (CFA, Imject Freund's complete adjuvant, Pierce, IL, U.S.A.) (n=30) was sensitized with 100 µg of D. farinae (Bencard, Worthing, U.K.) subcutaneously at the base of tail in CFA. The CFA group was also given an intraperitoneal dose of 300 ng of purified pertussis toxin (Sigma-Aldrich, St. Louis, MO, U.S.A.) 24 and 72 hr after the first immunization. Seven days later, the CFA group received again the same amount of D. farinae in incomplete Freund's adjuvant (Imject Freund's incomplete adjuvant, Pierce). Simultaneously, 25 µg D. farinae dissolved in 50 µL PBS was administered six times; it was inhaled through the nose once a week for 6 weeks. Seven weeks after the immunization, the CFA 7 group (n=15) was treated with 25 µg of D. farinae by intranasal inhalation for 3 consecutive days and had the airway responsiveness measured, and 24 hr later, bronchoalveolar lavage and histological study were performed. The control 7 group (n=15) was treated with PBS only. The CFA 12 group (n=15) was treated with intranasal inhalation of 25 µg of D. farinae from 7 weeks after the immunization to 11 weeks, 3 times a week. Twelve weeks after the immunization, the CFA 12 group inhaled intranasal 25 µg of D. farinae for three consecutive days; this was followed by measurement of airway responsiveness, and 24 hr later, bronchoalveolar lavage and histological study were performed. The control 12 group (n=15) was treated with PBS intranasal inhalation of PBS instead of D. farinae (Fig. 1).
Enzyme-linked immunosorbant assay (ELISA) detected the total IgE level as follows: 100 microliters of anti-mouse IgE captured mAb (clone R35-72; Pharmingen, San Diego, CA, U.S.A.) were added to each well, plated and incubated at 4℃, for overnight. After blocking with PBS+10% fetal calf serum, mouse sera were incubated overnight at 4℃ and antibody binding was assessed by the addition of 100 microliters of HRP-conjugated anti-mouse IgE detection mAb (clone R35-118; Pharmingen) overnight at 4℃. After the addition of the enzyme substrate, plates were read at 492 nm in an ELISA reader (Bio-Rad, Richmond, CA, U.S.A.). The purified mouse anti-IgE antibody (Pharmigen) was used for the total IgE standard.
ELISA determined D. farinae specific IgG1 as follows (15). Fifty microliters of D. farinae (5 µg/mL in 0.1 M carbonate buffer, pH 9.6) were dispensed into each well of a polystyrene micro titer plate (Costar, Cambridge, MA., U.S.A.) and incubated overnight at 4℃. The antigen-coated plates were washed three times with 0.05% PBS-Tween 20 buffer (washing buffer). Then they were incubated with mice sera overnight at 4℃. The plates were washed five times with washing buffer and incubated with peroxidase-conjugated anti-mouse IgG1 antibody (Sigma, St. Louis, MO, U.S.A.) overnight at 4℃, and then washed further five times before adding citric acid-phosphate buffer (pH 5.0) containing 0.15 mg/mL of O-phenylenediamine (Sigma). Color was developed at room temperature, and the reaction was stopped with 2.5 M sulfuric acid, and measured at 492 nm (Bio-Rad, Richmond, CA, U.S.A.). The Antibody Production Units of antigen-specific antibodies were determined by comparison of the plot of absorbance versus dilutions of the sample to that of the standard versus its concentration.
At the seventh week and twelfth week of the experiment, each group was treated with HDM or PBS intranasal inhalation. For the last treatment, the mice were placed in a barometric plethysmographic chamber (OCP 2000, Allmedicus, Anyang, Korea). Methacholine was used at the concentrations of 3.125 mg/mL, 6.25 mg/mL, 12.5 mg/mL, and 50 mg/mL. Three milliliters of each concentration were inhaled for 3 min as an aerosol prepared by an Ultra-Neb (3650p, DeVilbiss, Pennsylvania, PA, U.S.A.) and the Penh (enhanced pause) value for 3 min was measured. The airway responsiveness was expressed as Penh, which was calculated as follows, according to the manufacture's protocol:
Penh=(Expiratory time/Relaxation time-1)×(Peak expiration flow/Peak inspiration flow)
Results were expressed as the percentage increase in Penh after challenge with each concentration of methacholine, where baseline Penh (after saline challenge) is expressed as 100%.
Twenty-four hours after the assessment of the airway responsiveness, the left major bronchus was tied with a string, inserted with a 22-gauge needle, and the bronchoalveolar lavage fluid was obtained by infusion and collection of 0.4 mL PBS repeated for 3 times. The bronchoalveolar lavage fluid was centrifuged at 2,000 rpm for 7 min at 4℃, and the supernatant was stored at -70℃. The pellet was resuspended with 1 mL PBS containing 10% FBS (Gibco, Grand Island, NY, U.S.A.), and the total cell number was counted by mixing the 100 µL supernatant with 300 µL trypan blue (Gibco, Grand Island, NY, U.S.A.) and using a hematocytometer. The differential calculation of alveolar macrophage, eosinophils, lymphocytes, and neutrophils was carried out by adjusting the cell number to 1×106 cells/mL, preparing slides with a cytocentrifuge (Shandon Inc. Pittsburgh, PA, U.S.A.), staining with Diff-Quik, and counting at least 500 cells under 400×magnification.
The concentration of IL-13, IL-4, IL-10, IFN-γ, and TGF-β (R&D system, Minneapolis, MN, U.S.A.) in the bronchoalveolar lavage fluid was measured using ELISA kits. The minimum detectable concentration was 5, 2, 4, 2, and 7 pg/mL, respectively.
The left side of the lung that did not have bronchoalveolar lavage was resected, fixed immediately in 4% paraformaldehyde, and tissue sections were prepared. To examine the increase of goblet cells in the epithelium of the bronchus, periodic acid-Schiff (PAS) staining was performed. The degree of change was assessed by the application of a 5-point scoring system, which classified changes according to the proportion of goblet cells in the epithelium of the entire bronchus. Grade 0, was the absence of goblet cells; grade 1, less than 25%; grade 2, 25-50%; grade 3, 50-75%, and grade 4, 75-100% or the obstruction of the bronchus by mucous (16). To examine the degree of subepithelial fibrosis and the peribronchial smooth muscle layer, Masson's trichrome staining was performed. The stained area of the subbasement membrane due to fibrosis and the stained area due to smooth muscle were measured (17). For all bronchi studied, the average value was obtained, based on the circumference of the basement membrane, in two or three equivalent-sized and round-shaped bronchi. All measurements were performed with the computerized image analyzer program (BX50, Olympus, Tokyo, Japan).
The data were presented as the mean±standard deviation. The statistical analysis of parameters studied was performed by the independent sample t-test using the software for an IBM-PC (SPSS for Windows version 10.0). A p value less than 0.05 was considered statistically significant.
From 3 weeks after priming, the total serum IgE response in the CFA group (4.28±0.04 µg/mL) was significantly higher than that in the control (p<0.01). The high levels were maintained until 6 weeks (5.38±0.0 µg/mL), 9 weeks (6.09±0.06 µg/mL), and 12 weeks (6.11±0.06 µg/mL) after induction by intranasal HDM (Fig. 2A). In regard to the D. farinae specific IgG1 response, indirectly representative of effects of IL-4, 3 weeks after the immunization injection, it was higher in the CFA group (61.86±1.68×106 APU) than in the control group (23.26±2.10×106 APU) (p<0.01). The increase lasted until 6 weeks (94.46±2.10×106 APU), 9 weeks (95.58±2.30×106 APU), and 12 weeks (117.74±1.80×106 APU) (Fig. 2B).
The total cell number in the broncoalveolar lavage fluid of the CFA group 7 weeks after the immunization (20.24±4.00×105/mL) and after 12 weeks (12.40±7.15×105/mL) was significantly higher than in the control group (4.28±0.74×105, 3.99±0.83×105/mL) (Fig. 3). The number of eosinophils in the CFA group after 7 weeks (5.51±1.96×105/mL) and 12 weeks (1.64±1.40×105/mL) was increased. Similarly, in regard to neutrophils, they were higher in CFA 12 group (2.22±1.51×105/mL) than in the CFA 7 group (0.154±0.06×105/mL) (p<0.05) (Fig. 3).
To examine the changes in cytokines, the presence of cytokines in the supernatant of the bronchoalveolar lavage fluid was measured. At 7 weeks after the immunization, the CFA 7 group had higher levels than the control groups, IL-4 (33.04±10.88 pg/mL vs. 13.16±0.56), IL-13 (16.78±2.44 pg/mL vs. 8.47±0.74), and TGF-β (16.29±6.80 pg/mL vs. 4.58±0.45) (p<0.05). Similarly, at 12 weeks, increases of IL-4 (49.70±17.64 pg/mL vs. 13.61±0.82), IL-13 (75.20±29.22 pg/mL vs. 7.82±0.08), and TGF-β (37.68±13.88 pg/mL vs. 6.26±1.06) (p<0.05) were detected. Among cytokines, only IL-13 was significantly increased with increase of exposure time (CFA 7 vs. CFA 12, p<0.05). By contrast, IL-10 and IFN-γ were comparable to the control levels (Table 1).
To assess the clinical characteristics of asthm and the presence or absence of airway responsiveness, the bronchial provocation test using methacholine was performed. It was higher in the CFA groups than in the control groups in response to all methacholine concentrations. There was no difference according to the exposure time (Fig. 4).
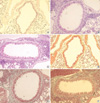
The results from the PAS staining of lung tissues showed that there was no increase in goblet cells in the control group. However, an increase was clearly detected in the CFA group (Fig. 5A-C). For the Masson's trichrome staining, an increase in subepithelial fibrosis and thickening of the peribronchial smooth muscle was detected in the CFA group compared to the control 12 group (Fig. 5D-F). Measuring the increase of goblet cells by applying the 5-point scoring system, an increase was detected in the CFA 7 and 12 groups; however, the degree was not changed with the exposure time. By contrast, an increase was not detected in the control group (Fig. 6A). For the Masson's trichrome staining, at seventh week of the experiment, there was a significant increase in subepithelial fibrosis and the thickening of the peribronchial smooth muscle layer in the CFA 7 group compared to the controls. After 12 weeks, the increase in the CFA 12 group continued (13.88±6.24×104 µm2 vs. 6.74±3.44×104 µm2, p<0.05) (Fig. 6B). However, the change of the subepithelial fibrosis and the peribronchial smooth muscle areas did not change with time.
Chronic inflammation of the airway causes structural abnormalities and the development of airway remodeling. Among these changes, the hyperplasia of goblet cells and structural alterations of the subbasement membrane induce functional impairment resulting in the appearance of the typical clinical symptoms from bronchial hyperreactivity and airway constriction (18, 19). Clinically, although early prevention or treatment of airway remodeling may facilitate improvements in asthma symptoms, specific interventions that improve airway remodeling are scarce at present (20). Thus, in order to develop the therapeutics that can treat the airway remodeling morphology, characterization of the mechanism mediating airway remodeling is required. The development of a murine airway-remodeling model would help reach this goal. Here, we present a murine experimental system with repeated exposure to the common allergen, HDM, for 12 weeks, and present findings of airway remodeling factors, hypertrophy of smooth muscles, increase in the degree of fibrosis in mucous membranes, and hyperplasia of goblet cells. Regarding the airway responsiveness and the bronchoalveolar lavage fluid, HDM-exposed mice demonstrated that the number of eosinophils and other allergic inflammatory reactions were enhanced and severe airway hyperreactivity was observed in response to methacholine.
Some experiments, however, have reported that inhalation of ovalbumin for 12 weeks weakens the degree of airway remodeling and observations of airway responsiveness were lost (21). Clinically, ovalbumin is a rare allergen. It cannot induce chronic airway inflammation readily by itself, and induces tolerance rather than priming in most cases (14). In this study, allergic bronchial asthma induced by the repeated or continuous exposure to aeroallergens resulted in a chronic inflammatory condition, using a common clinical allergen, HDM. This exposure resulted in the maintenance of the specific characteristic of bronchial asthma, airway remodeling.
Airway remodeling is a repair phenomenon in chronic airway inflammation and damage and involves various mediators. The mechanism to maintain the normal conditions is disturbed resulting in the appearance of hypertrophy of smooth muscles, the fibrosis of the subbasement membrane, and hyperplasia of goblet cells to name a few of the altered factors. In addition, the cytokines that are involved in the immune response of the type 2 helper cells, such as IL-13, IL-4 and TGF-β, mediate their effects on the epithelial cells in the airway. These immune mediators result in hyperplasia of goblet cells, and simultaneously induce fibrosis in the subbasement membrane, causing alteration of the components of the extracellular matrix (5, 6, 17, 22). The evaluation of cytokines in the bronchoalveolar lavage fluid performed to assess the effect of such mediators showed that after 7 weeks and 12 weeks, the concentrations of IL-4, IL-13, and TGF-β increased continuously (Table 1). These findings are consistent with prior reports demonstrating that among growth factors, TGF-β secreted by the epithelial cells in the airway induces hypertrophy of smooth muscle cells and fibrosis in the subbasement membrane by altering myofibroblasts. In addition, the type 2 helper immune response cytokines IL-4 and IL-13 are produced. The interaction between TGF-β and the cytokines results in chronic inflammation and the acceleration of airway remodeling (6).
Concerning the proportion of inflammatory cells in the bronchoalveolar lavage fluid, in CFA 7 and 12 groups, the total cell numbers as well as eosinophils were increased. As the exposure to HDM continued, in the CFA 12 group, an increase in neutrophils was detected. Simultaneous assessment of the degree of airway remodeling showed that hyperplasia of goblet cells, subepithelial fibrosis, and the hypertrophy of the peribronchial smooth muscle were observed until 12 weeks, suggesting that eosinophils play an important role in airway remodeling.
Eosinophils released by TGF-β primarily, increase the synthesis of fibronectin, collagen I, and III, and thus facilitate the progression of airway remodeling (23). The interaction of the immune response of the Th2 helper cells and the bronchial epithelial cells stimulates the release of various cytokines resulting in airway remodeling (6). Thus, it is believed that the increase of eosinophils maintains the Th2 immune response and simultaneously, through TGF-β, facilitates the progression, or at least the maintenance, of the airway remodeling.
In addition, in the airway or blood of bronchial asthma patients, neutrophils account for over 50% of cells that are involved in the thickening of the subbasement membrane and cells positive for TGF-β (24). Neutrophils have been reported to play an important role in the degranulation of goblet cells (25), which suggests an important role for neutrophils in airway remodeling. However, in animal studies, from 2 hr to 48 hr after a challenge with allergens, neutrophils have been reported to be temporarily increased in the bronchoavleolar lavage fluid (26).
It has been reported that such a response is the result of a challenge with a large allergen bolus in the laboratory; these observations were not reported with chronic exposure to a small amount of allergen where neutrophils were increased continuously (27). In addition, it has been reported that neutrophils induce hyperplasia of goblet cells and the progression of subepithelial fibrosis by stimulating the airway epithelial cells through the release of oxidants resulting in the release of epidermal growth factor and TGF-β, which stimulates the infiltration of neutrophils in the airway by the release of IL-8 (27). In our study, in the 12 week group, the number of neutrophils in the CFA 12 group was increased even though the number of eosinophils in the CFA-12 group was reduced more than in the CFA 7 group. IL-13 was increased in the supernatant of the bronchoalveolar lavage fluid, and the high level of IL-4 and TGF-β was maintained. This suggests the involvement of neutrophils in airway remodeling with increased exposure time.
It was observed that the total serum IgE antibody response induced by the type two helper cell responses was increased in the CFA group until 6 weeks and continued for 12 weeks, and the D. farinae specific IgG1 antibody response, which is influenced by IL-4, increased with the increase of the exposure time to allergens.
IFA is a surfactant composed of paraffin oil containing mannide mono-oleate. It has been reported that CFA is a potent adjuvant as it contains heat-inactivated mycobacteria, lengthens the period of the presence of allergens when administered together, and delivers them efficiently to the immune system. IFA induces the type 2 helper cell response and thus increases antibody production (28). Here, our data demonstrate that the application of such an adjuvant together with HDM allows maintenance of the type 2 helper response continuously.
In conclusion, our data demonstrate that repeated exposure to HDM maintains continuously airway-remodeling factors, hypertrophy of smooth muscles, increase of fibrosis in the subbasement membrane, hyperplasia of goblet cells, and the allergic inflammatory response such as airway hyperreactivity. In addition, in the bronchoalveolar lavage fluid, the increase of IL-4, IL-13, and TGF-β was maintained, and this may play an important role in the airway remodeling process. In addition, neutrophils increased as the exposure time increased, which suggests that neutrophils may also play an important role in the etiology of chronic bronchial asthma.
Figures and Tables
Fig. 1
Immunization scheme and the time course for house dust mite (HDM) sensitization and provocation. Penh, the enhanced pause value.

Fig. 2
Time course of total serum IgE (A) and D. farinae specific IgG1 (B) levels following chronic challenges. Data represent means±SE (n= 15), *p<0.01, the CFA group compared with the control group.

Fig. 3
The number of inflammatory cells in bronchoalveolar lavage fluid (BALF) at 7 and 12 weeks after systemic immunization. Data represent means±SE (n=15), *p<0.05, the CFA group compared with the control group. †p<0.05, the CFA 12 group compared with CFA 7 group.

Fig. 4
Analysis of airway responsiveness to methacholine in each group at 6 and 12 weeks after systemic immunization of house dust mites. *p<0.01, the CFA group compared with the control group.

Fig. 5
Histological analysis of lung sections at 12 weeks after systemic immunization with house dust mites. The lung sections stained with Periodic acid-Schiff (A-C) and Masson's trichrome (D-F). Slides were photographed at ×100 magnification. (A, D) Control 12 group, (B, E) CFA 7 group, and (C, F) CFA 12 group.
ACKNOWLEDGMENTS
We would like to thank Jung Me Lee and Jin Suk Kim for technical help throughout the experiments, and Sang Hee Park at Holy Family Hospital who performed histological studies.
References
2. Boulet LP, Turcotte H, Laviolette M, Naud F, Bernier MC, Martel S, Chakir J. Airway hyperresponsiveness, inflammation, and subepithelial collagen deposition in recently diagnosed versus long-standing mild asthma: influence of inhaled corticosteroids. Am J Respir Crit Care Med. 2000. 162:1308–1313.
3. Laprise C, Laviolette M, Boutet M, Boulet LP. Asymptomatic airway hyperresponsiveness: relationships with airway inflammation and remodeling. Eur Respir J. 1999. 14:63–73.
4. Fish JE, Peters SP. Airway remodeling and persistent airway obstruction in asthma. J Allergy Clin Immunol. 1999. 104:509–516.

5. Kips JC, Pauwels RA. Airway wall remodelling: does it occur and what does it mean? Clin Exp Allergy. 1999. 29:1457–1466.

6. Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: New insights. J Allergy Clin Immunol. 2003. 111:215–225.
7. Macklem PT. A theoretical analysis of the effect of airway smooth muscle load on airway narrowing. Am J Respir Crit Care Med. 1996. 153:83–89.

8. Solway J, Fredberg JJ. Perhaps airway smooth muscle dysfunction contributes to asthmatic bronchial hyperresponsiveness after all. Am J Respir Cell Mol Biol. 1997. 17:144–146.

9. Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997. 156:737–743.
10. Cox G. The role of neutrophils in inflammation. Can Respir J. 1998. 5:Suppl A. 37A–40A.
12. Swirski FK, Sajic D, Robbins CS, Gajewska BU, Jordana M, Stampfli MR. Chronic exposure to innocuous antigen in sensitized mice leads to suppressed airway eosinophilia that is reversed by granulocyte macrophage colony-stimulating factor. J Immunol. 2002. 169:3499–3506.

13. Kim JS, Lee JM, Kim SJ, Lee SY, Kwon SS, Kim YK, Kim KH, Moon HS, Song JS, Park SH. The preventive effect by induction of oral tolerance in a mouse model of allergic asthma. Tuberc Respir Dis. 2004. 57:425–433.
14. Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004. 169:378–385.

15. Wolfowicz CB, HuangFu T, Chua KY. Expression and immunogenicity of the major house dust mite allergen Der p 1 following DNA immunization. Vaccine. 2003. 21:1195–1204.

16. Tanaka H, Masuda T, Tokuoka S, Komai M, Nagao K, Takahashi Y, Nagai H. The effect of allergen-induced airway inflammation on airway remodeling in a murine model of allergic asthma. Inflamm Res. 2001. 50:616–624.

17. Ellis R, Leigh R, Southam D, O'Byrne PM, Inman MD. Morphometric analysis of mouse airways after chronic allergen challenge. Lab Invest. 2003. 83:1285–1291.

18. Leigh R, Ellis R, Wattie J, Southam DS, De Hoogh M, Gauldie J, O'Byrne PM, Inman MD. Dysfunction and remodeling of the mouse airway persist after resolution of acute allergen induced inflammation. Am J Respir Cell Mol Biol. 2002. 27:526–535.
19. Fahy JV. Remodeling of the airway epithelium in asthma. Am J Respir Crit Care Med. 2001. 164:S46–S51.

20. Djukanovic R. Airway inflammation in asthma and its consequences: Implications for treatment in children and adults. J Allergy Clin Immunol. 2002. 109:S539–S548.

21. Shinagawa K, Kojima M. Mouse model of airway remodeling: strain differences. Am J Respir Crit Care Med. 2003. 168:959–967.
22. Kim SJ, Lee SY, Park YK, Kim MH, Kim SC, Kwon SS, Kim YK, Kim KH, Moon HS, Song JS, Park SH. The relationship between airway remodeling and transforming growth factor-β 1 and basic fibroblast growth factor expression in bronchoalveolar cells from asthmatics. J Asthma Allergy Clin Immunol. 2002. 22:532–539.
23. Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active Transforming Growth Factor-β 1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997. 100:768–776.
24. Chu HW, Trudeau JB, Balzar S, Wenzel SE. Peripheral blood and airway tissue expression of transforming growth factor β by neutrophils in asthmatic subjects and normal control subjects. J Allergy Clin Immunol. 2000. 106:1115–1123.
25. Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000. 164:1546–1552.

26. Taube C, Dakhama A, Takeda K, Nick JA, Gelfand EW. Allergen-specific early neutrophil infiltration after allergen challenge in a murine model. Chest. 2003. 123(Supple 3):410S–411S.

27. Corbel M, Caulet-Maugendre S, Germain N, Lagente V, Boichot E. Enhancement of gelatinase activity during development of subepithelial fibrosis in a murine model of asthma. Clin Exp Allergy. 2003. 33:696–704.

28. Billiau A, Matthys P. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001. 70:849–860.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download