Abstract
The aim of this study was to follow up the changes in the number of motor units according to the Brünnstrom stage through a motor unit number estimation of the F-wave (F-MUNE) after a stroke, and to identify the functional significance of F-MUNE. Twenty-five patients (15 men, 10 women) with a first unilateral stroke were recruited. The maximal M-potential was evoked by the supramaximal stimulation of the median nerve at the wrist, and the maximal stimulation intensity was determined on both hemiplegic and unaffected hands. The reproducible all-or-none F-wave was evoked in 30% of the maximal stimulation intensity and was constantly stimulated at that level. The prototypes of the F-wave were chosen, and the values of F-MUNE were calculated by dividing the amplitude of the maximal M-potential by the mean amplitude of the F-prototype. The changes in F-MUNE were compared according to the progression of the Brünnstrom stage and correlated with those of the functional scales. The mean motor unit numbers decreased significantly in the hemiplegic side compared with the unaffected side. According to the progression of the Brünnstrom stage, the values of F-MUNE were reduced significantly by increasing the amplitude and recruitment of the F-prototype, and the functional scores also improved. These results show that the F-MUNE equation did not show a functional recovery-related increase in stroke patients.
Although a prior history of stroke, older age, urinary and bowel incontinence, and visuospatial deficits have been identified as poor prognostic indicators of an independent function (1), there is no established objective and reproducible indicator of the stroke outcome. Fisher et al. (2) reported that the H reflex and F wave studies provided information regarding the amount of excitable motor neurons but there is no definite evidences as to how well they work. On the other hand, Drory et al. (3) reported that the serial changes in the F wave parameters (persistence, maximal and average duration, amplitude, and area) in acute stroke patients were significantly lower on the hemiplegic side. McComas et al. (4), in a study of 27 hemiplegic patients, reported that the functioning motor units from the foot intrinsic muscles were observed to decrease by 50% on the hemiplegic side within two months of a cerebrovascular accident. In the chronic stage, the average F wave amplitude and area on the hemiplegic side increased, which is similar to or larger than those of the unaffected side. However, the correlation with the muscle strength and the changes in tone was poor (3). Therefore, they concluded that the spinal motor neuron excitability was low during the acute stage, and would tend to normalize or even exceed the unaffected side in the chronic stage. Higashi et al. (5) used a new method to compare the excitability of the motor neuron pools of spastic hemiplegic patients; the ratio of the developmental slope of the H-reflex to the slope of the M response. The lack of marked augmentation of the gamma-activity in spastic patients suggests that there was higher alpha motor neuron pool excitability in the monosynaptic reflex arc of the spastic side in hemiplegic patients than in unimpaired subjects (6). Recently, Sohn and Han suggested the clinical utility of an F-wave evaluation in stroke patients (7).
A motor unit number estimation (MUNE) is important for the prognostic factor of the motor recovery (8). The antecedent F-wave motor unit number estimation (F-MUNE) study, one of the easily accessible methods, is performed by computer analysis with special software and was taken as the negative peak area for MUNE study by Stashuk et al. (9). However, the use of the negative amplitude for MUNE is acceptable for practical simplicity, not requiring special computer analysis. Hara et al. (10) used the F wave method to reduce the "alteration effect", which causes calculation errors in electrophysiological MUNE methods such as the incremental method. Doherty and Brown (11) reported that the degree of variability in the average surface motor unit action potentials (S-MUAPs) was substantially reduced, after which the average value remained relatively stable. A practical F-MUNE recently showed that even submaximal stimuli, in which 10% to 30% of the stimulation intensity that evokes the maximal M-potentials, can evoke a sample of S-MUAPs in the F-response. For this, Hara et al. (10) confirmed the stability and reproducibility of the F-MUNE method in the same bilateral muscle of healthy volunteers after acquiring at least 10 S-MUAPs or more based on the total sum of the average amplitude of submaximal stimuli-evoked S-MUAPs satisfactorily reflecting the number of functional motor units. They used the F-MUNE method, where the MUNE equals the negative peak amplitude of the maximum M-potential divided by the average negative peak amplitudes of the S-MUAPs in the submaximal-stimulated F-response on the abductor pollicis brevis (APB) muscle. They suggested that a decrease in the F-MUNE in hemiplegic limb might reflect the trans-synaptic degeneration of motor neurons in patients with moderate-to-severe hemiplegia. However, there have been no prospective studies on the longitudinal changes in F-MUNE in the same stroke patients over a sufficiently long duration to investigate the subsequent enlargement of the remaining motor units with sprouting morphologically suggested by Benecke et al. (12) and Dattola et al. (13).
This study investigated the prospective changes in the F-MUNE after a stroke according to the Brünnstrom stage, how these changes correlate with the clinical state, and the functional significance of F-MUNE.
Twenty-five patients (15 men, 10 women), who had suffered from first unilateral stroke with consequent motor deficits, were examined with given informed consent. The mean age was 60.9±10.4 yr, and the duration from the onset of hemiplegia to the initial examination was within a week. The mean period of follow-up study was 11.32±9.7 weeks. The study was confined to patients with a cerebral infarction on the middle cerebral artery that was diagnosed by brain magnetic resonance imaging (MRI). The patients with cervical spondylosis, diabetes mellitus, or peripheral neuropathy according to their clinical history, physical and neurological examination, and/or electrophysiological study were excluded. All patients had moderate-to-severe hemiplegia with Brünnstrom stage 1 in the upper extremities including hand paralysis. Each patient's motor recovery stage was assessed using the six Brünnstrom stages judged by clinical observation (14) (Table 1). We checked the neurological examination five days a week for 2 months and then followed up every week for 9 months. The number of cases at each Brünnstrom stage were as follows: 25 cases at stage I, 13 cases at stage II, 17 cases at stage III, 9 cases at stage IV, 8 cases at stage V, and 8 cases at stage VI. Seventeen patients had not been progressed until the Brünnstrom stages VI.
An electrophysiological examination was performed using a Viking 4 Plus (Nicolet®, U.S.A., 1999) electrodiagnostic machine with the patients lying supine in a warm room maintained at 22-24℃. The patients were encouraged to relax in order to prevent voluntary activity interfering with the data collection. A 1.1 cm-diameter Ag-AgCl surface electrode was used for the MUNE. The active recording electrode was positioned over the anatomical midpoint of the APB muscle, and the reference recording electrode was positioned over the metacarpal phalangeal (MP) joint of the thumb. A ground electrode was positioned between the stimulation and active recording electrode.
The maximum M-potential (MMP) on the APB muscle was taken by the supramaximal stimulation of the median nerve at the wrist, and the maximal stimulation intensity (MSI) was determined on the both hemiplegic and unaffected hands (10). The stimulus current intensity was initiated from the minimal level, where neither the F-wave nor MMP was evoked, and adjusted to evoke the reproducible all-or-none F-wave on 30% of MSI. After the bipolar stimulator had been stabilized at the wrist, successive stimulation was performed at that level (150 repetitions). The signals detected were displayed using a band pass of 2 Hz to 2 kHz. The stimulation frequency was 1 Hz, and the duration was 200 milliseconds. The entire product of the individual F-waves was collected, and the prototypes of the F-wave that had two or more F-waves with the same latency, amplitude and shape were chosen (Fig. 1). In order to determine this, a candidate F-prototype was selected and visually compared individually with every other remaining response in the raster using the screen and printing. Identical responses were grouped and removed from the list to be analyzed later, and a new candidate F-prototype was selected and compared in the same manner. The average negative peak amplitude of the F-prototype was calculated from the selected F-prototypes. The F-MUNE value was calculated by dividing the negative peak amplitude of MMP by the average negative peak amplitude of F-prototypes. The serial changes in F-MUNE was investigated according to the progression of the Brünnstrom stage, and the functional recovery by Functional independence measure (FIM) and modified Barthel index (MBI) (15).
Statistical analyses were performed. The mean values along with their standard deviations are presented. An independent t-test was used to compare the mean motor unit numbers of the F response from the hemiplegic side with those from the unaffected sides among the patients, and repetitive measure analysis was used to investigate the significance of the serial changes in the F-MUNE according to the progression of the Brünnstrom stage and functional recovery.
The mean numbers of motor unit F-waves on the unaffected side and the hemiplegic side were 35.4±11.4 and 23.9±12.5. The mean number of motor unit F-waves in the hemiplegic side was significantly smaller than in the unaffected side (p=0.01).
The peak amplitude of MMP in the hemiplegic side was significantly smaller than in the unaffected side (p=0.000), but there were no significant changes according to the progression of the Brünnstrom stage on both sides (p>0.05, Fig. 2).
The functional scores by the FIM and MBI were significantly improved according to the progression of the Brünnstrom stage (p=0.000, Fig. 3).
The frequency of the F-prototypes was increased progressively (p=0.000) according to the Brünnstrom stage of motor recovery. However, the F-MUNE was significantly lower according to the progression of Brünnstrom stage (p=0.000, Fig. 4) : 46.6±2.1 at stage 1, 32.3±3.2 at stage 2, 23.3±2.5 at stage 3, 16.6±1.3 at stage 4, 14.0±1.9 at stage 5 and 11.1±1.3 at stage 6.
The F wave, which was first described by Magladery and McDougal (16) in 1950, is the motor response produced by supramaximal antidromic stimulation of the motor nerve fibers without a synaptic component. The persistence of F waves recorded after a train of antidromic stimuli indicates the state of excitability of the motor neurons in the neuronal pool examined (17). In addition to the extensive reports on the F response in peripheral nervous system disorders, there are few reports on the influence of the central neurological processes (18). Since all central motor dysfunctions must ultimately involve motor neuron discharge, the F response can be a valuable tool for investigating the central excitability state of motor neurons as well as in gaining a better understanding motor system physiology in upper motor neuron disorders (7, 19).
Kondo et al. (20) reported that the degree of degeneration of the lateral corticospinal tracts appeared to be parallel to that of fiber loss in the ventral roots in stroke patients. In addition, Spaans and Walts (21) concluded that lesions of the central nervous system might cause a dysfunction of anterior horn cells, which leads to axonal degeneration in the form of a dying-back process. This dysfunction is temporary, so that axonal regeneration and reinnervation can soon take place. Fierro et al. (22) suggested that the initial stages of a reduced spinal motoneuron excitability was present in the early phase of stroke, followed by increased motor neuron excitability within 90 days. They also suggested that the increase in F/M% observed in chronic hemiplegia might be due to the lowering of the MMP as a result of disuse atrophy of hemiplegic limbs. Indeed, a functional and structural rearrangement characterized by an increased number of type 1 fibers and type 2 atrophy has been observed in muscle spasticity. Similar changes in non-hemiplegic limbs were observed possibly because of the decrease in general physical activity.
Surprisingly, the amplitudes of MMP on the paretic side were significantly smaller than those of the sound side, but there were no significant differences according to the progression of the Brünnstrom stage on each side. This is because of the decreased activity of the paretic muscles with secondary atrophy. This finding has rarely been mentioned in previous studies (22). There are some reports that the decrease in the hemiplegic side M-potential reflects the decrease in the number of motor units (18). In our results, the peak amplitude of MMP in the hemiplegic side decreased until Brünnstrom stage 4 was reached, and then tended to increase. This suggested that the patients activated selectively their muscles along the neurological recovery, and so the number of motor units progressively increased after Brünnstrom stage 4.
Frequently, a larger M-potential than the initial M-response was observed in our patients on the follow-up F-MUNE study. This suggests hypertrophy and sprouting of the remaining motor neuron after trans-synaptic degeneration. Moreover, the F-MUNE equation should be taken into account by adding "variables" associated with the hypertrophy and sprouting of the remaining motor neuron after trans-synaptic degeneration, i.e., not only the "peripheral down-regulation effect" but also the "peripheral recovery effect". If this is not done, the F-MUNE equation might result in misinterpretation of the theoretical interpretation of motor recovery.
The functional scales, such as FIM and MBI, were used to objectively measure the degree of functional improvement according to the Brünnstrom stage. Two neurological mechanisms have been implicated in the generation of the motor performance (23). One is the number of motor units recruited, and the other is the modulation of the firing rate of a single motor unit, which had already been recruited. The number of motor units voluntarily recruited was significantly lower in spastic patients (10). Using the F-MUNE method, the sum of the average submaximal stimuli-evoked S-MUAPs of 10 or more motor units might not equal the supramaximal stimuli-evoked MMP representative of the whole functional motor neuron excitability, and that the F-MUNE equation does not consider the changes in the motor neurons after trans-synaptic degeneration. Furthermore, most stroke patients show relatively stable and constant MMP and MSI. This suggests that the F-MUNE method had limitations for the general use of MUNE, which reflects the number of functional motor units. The mean number of F-waves from the motor units is significantly lower in the hemiplegic side than in the unaffected side, as suggested by the trans-synaptic degeneration theory of alpha motor neurons in the hemiplegic side on initial examination within 7 to 10 days after stroke. However, as the Brünnstrom stage and functional scores were improved, the values of F-MUNE were significantly reduced, which is in contrast to previously reported suggestions, due to the increased amplitude and recruitment of the F-prototype. Hence, our longitudinal study shows that F-MUNE did not indicate a functional recovery-related increase in stroke patients.
This study revealed that the F-MUNE equation does not show the functional recovery-related increase in stroke patients. Therefore, the previously well-known F-MUNE statistics may not be fairly applicable to the stroke patients, in that the concept of the hypertrophy and sprouting of the remaining motor neurons after trans-synaptic degeneration, and the relatively unchangeable amplitude of MMP and MSI. In addition, this equation should be modified or corrected to take into account the motor recovery because of the paradoxically decreasing tendency along with the progression of Brünnstrom stage and functional recovery. Currently, there are no longitudinal-survey reports on the correlation between the estimation of the number of motor units showing an F response and the functional recovery in stroke patients.
Figures and Tables
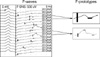 | Fig. 1The prototypes of the F-wave, identically matched two or more F-waves with the same latency, amplitude, and shape were selected among the entire products of individual F-waves. |
 | Fig. 2The peak amplitude of the maximal M-potential (MMP) in the hemiplegic side was significantly smaller than in the unaffected side (p=0.000, between groups), but there was no significant changes according to the progression of the Brünnstrom stage on both sides (p>0.05, within groups). Repetitive measure analysis was used to examine the significance of the serial changes in the MMP according to the progression of the Brünnstrom stage. |
 | Fig. 3The functional scores by the functional independence measure (FIM) and modified Barthel index (MBI) were significantly improved according to the progression of the Brünnstrom stage (p=
0.000, repetitive measure analysis). |
References
1. Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Støier M, Olsen TS. Outcome and time course of recovery in stroke. Part 1: Outcome. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995. 76:399–405. .
2. Fisher MA, Shahani BT, Young RR. Assessing segmental excitability after acute rostral lesions: II. The blink reflex. Neurology. 1979. 29:45–50.
3. Drory VE, Neufeld MY, Korczyn AD. F wave characteristics following acute and chronic upper motor neuron lesions. Electromyogr Clin Neurophysiol. 1993. 33:441–446.
4. McComas AJ, Sica REP, Upton AR, Aguilera N, Currie S. Motoneuron dysfunction in patients with hemiplegic atrophy. Nat New Biol. 1971. 233:21–23.
5. Higashi T, Funase K, Kusano K, Tabira T, Harada N, Sakakibara A, Yashimura T. Motoneuron pool excitability of hemiplegic patients: assessing recovery stages by using H-reflex and M response. Arch Phys Med Rehabil. 2001. 82:1604–1610.

6. Burke D. Critical examination of the case for or against fusimotor involvement in disorders of muscle tone. Adv Neurol. 1983. 39:133–150.
7. Sohn MK, Han SM. Facilitation of nerve conduction by distant muscle contraction in stroke patients. J Korean Acad Rehabil Med. 2005. 29:50–57.
8. McComas AJ. Invited review. Motor unit estimation: methods, results, and present status. Muscle Nerve. 1991. 14:585–597.

9. Stashuk DW, Doherty TJ, Kassam A, Brown WF. Motor unit number estimates based on the automated analysis of F-responses. Muscle Nerve. 1994. 17:881–890.

10. Hara Y, Akaboshi K, Masakado Y, Chino N. Physiological decrease of single thenar motor units in the F-response in stroke patients. Arch Phys Med Rehabil. 2000. 81:418–423.
11. Doherty TJ, Brown WF. The estimated numbers and relative sizes of thenar motor units as selected by multiple point stimulation in young and older adults. Muscle Nerve. 1993. 16:355–366.

12. Benecke R, Berthold A, Conrad B. Denervation activity in the EMG of patients with upper motor neuron lesions: time course, local distribution and pathogenetic aspects. J Neurol. 1983. 230:143–151.

13. Dattola R, Girlanda P, Vita G, Snatoro M, Robert ML, Toscano A, Venuto C, Baradello A, Messina C. Muscle rearrangement in patients with hemiparesis after stroke: an electrophysiological and morphological study. Eur Neurol. 1993. 33:109–114.

14. Brunnstrom S. Motor testing procedures in hemiplegia: Based on sequential recovery stages. Phys Ther. 1966. 46:357–375.

15. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989. 42:703–709.

16. Magladery JW, McDougal DB. Electrophysiological studies of nerve and reflex activity in normal man. I. Identification of certain reflexes in the electromyogram and the conduction velocity of peripheral nerve fibers. Bull Johns Hopkins Hosp. 1950. 86:265–290.
17. Fisher MA. Inhibition of motoneuron discharge by peripheral nerve stimulation: an F-response analysis. Muscle Nerve. 1991. 14:120–123.
18. Kimura J. . Other techniques to assess nerve function, Electrodiagnosis in diseases of nerve and muscle: principles and practice. 1983. 3rd ed. Philadelphia: F.A. Davis;216–217.
19. Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol. 2002. 113:936–943.

20. Kondo A, Nagara H, Tateishi J. A morphometric study of myelinated fibers in the fifth lumbar ventral roots in patients with cerebrovascular diseases. Clin Neuropathol. 1987. 6:250–256.
21. Spaans F, Wilts G. Denervation due to lesions of the central nervous system. An EMG study in cases of cerebral contusion and cerebrovascular accidents. J Neurol Sci. 1982. 57:291–305.
22. Fierro B, Raimondo D, Modica A. Analysis of F response in upper motoneuron lesions. Acta Neurol Scand. 1990. 82:329–334.
23. Yan K, Fang J, Turan b, Chira-Adisai W, Shahani BT. The use of fractional parameter in analyzing motor unit discharge pattern in stroke patients: a correlation with the functional independence measurement. Electromyogr Clin Neurophysiol. 2000. 40:3–9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download